Isolation and characterization of Human Mesenchymal Stromal Cells Derived from Placental Decidua Basalis; Umbilical cord Wharton's Jelly and Amniotic Membrane
- PMID: 25225519
- PMCID: PMC4163225
- DOI: 10.12669/pjms.305.4537
Isolation and characterization of Human Mesenchymal Stromal Cells Derived from Placental Decidua Basalis; Umbilical cord Wharton's Jelly and Amniotic Membrane
Abstract
Objective: Mesenchymal stromal cells (MSCs) are considered as an excellent source in regenerative medicine, but availability and ethical problems limited their routine use. Therefore, another available source with easy procedure and exempt from ethical debate is important. The purpose of this study is to isolate and characterize the MSCs from human placenta. The stromal cells were isolated from Placental Decidua Basalis (PDB-MSC), Umbilical cord Wharton's Jelly (WJ-MSC) and Amniotic Membrane (AM-MSC).
Methods: Full term human placentas (n=4), from cesarean section delivery were collected. Small fragments from different parts were cultures as explants. The immunophenotyping, mesodermal differentiation, growth kinetics and stemness gene expression was studied.
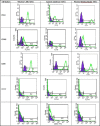
Results: The cultivated cells from three sources expressed CD44, CD105, and CD90. Gene expression of NANOG and OCT4 confirmed the undifferentiated state. The doubling-times for WJ-MSCs, PLC-MSCs and AM-MSCs, respectively, were 21±8h, 28±9h and 25±9h at passage three and 30±5h, 45±7h and 45±7h at passage tenth. The proliferative potential of WJ-MSCs tended to be higher than the other two sources.
Conclusion: The fetal derives stromal cells; especially the early passages of WJ-MSCs are available supplies for large scale production of MSC for using in clinical studies or research projects.
Keywords: Amnion; Mesenchymal stromal cells; Placenta; Umbilical cord.
Figures


References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
-
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. - PubMed
-
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. - PubMed
-
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. - PubMed
-
- Sarmadi VH, Tong CK, Vidyadaran S, Abdullah M, Seow HF, Ramasamy R. Mesenchymal stem cells inhibit proliferation of lymphoid origin haematopoietic tumour cells by inducing cell cycle arrest. Med J Malaysia. 2010;65:209–214. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
