Critical role of bicarbonate and bicarbonate transporters in cardiac function
- PMID: 25225601
- PMCID: PMC4160527
- DOI: 10.4331/wjbc.v5.i3.334
Critical role of bicarbonate and bicarbonate transporters in cardiac function
Abstract
Bicarbonate is one of the major anions in mammalian tissues and extracellular fluids. Along with accompanying H(+), HCO3 (-) is generated from CO2 and H2O, either spontaneously or via the catalytic activity of carbonic anhydrase. It serves as a component of the major buffer system, thereby playing a critical role in pH homeostasis. Bicarbonate can also be utilized by a variety of ion transporters, often working in coupled systems, to transport other ions and organic substrates across cell membranes. The functions of HCO3 (-) and HCO3 (-)-transporters in epithelial tissues have been studied extensively, but their functions in heart are less well understood. Here we review studies of the identities and physiological functions of Cl(-)/HCO3 (-) exchangers and Na(+)/HCO3 (-) cotransporters of the SLC4A and SLC26A families in heart. We also present RNA Seq analysis of their cardiac mRNA expression levels. These studies indicate that slc4a3 (AE3) is the major Cl(-)/HCO3 (-) exchanger and plays a protective role in heart failure, and that Slc4a4 (NBCe1) is the major Na(+)/HCO3 (-) cotransporter and affects action potential duration. In addition, previous studies show that HCO3 (-) has a positive inotropic effect in the perfused heart that is largely independent of effects on intracellular Ca(2+). The importance of HCO3 (-) in the regulation of contractility is supported by experiments showing that isolated cardiomyocytes exhibit sharply enhanced contractility, with no change in Ca(2+) transients, when switched from Hepes-buffered to HCO3 (-)- buffered solutions. These studies demonstrate that HCO3 (-) and HCO3 (-)-handling proteins play important roles in the regulation of cardiac function.
Keywords: AE1; AE2; NBCn1; SLC26; SLC4; Slc26a6.
Figures
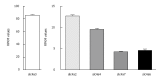
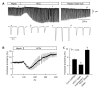
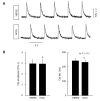
Similar articles
-
Molecular pathophysiology of SLC4 bicarbonate transporters.Curr Opin Nephrol Hypertens. 2005 Sep;14(5):495-501. doi: 10.1097/01.mnh.0000168333.01831.2c. Curr Opin Nephrol Hypertens. 2005. PMID: 16046910 Review.
-
Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart.J Physiol. 2004 Dec 15;561(Pt 3):721-34. doi: 10.1113/jphysiol.2004.077339. Epub 2004 Oct 21. J Physiol. 2004. PMID: 15498800 Free PMC article.
-
Cation-coupled bicarbonate transporters.Compr Physiol. 2014 Oct;4(4):1605-37. doi: 10.1002/cphy.c130005. Compr Physiol. 2014. PMID: 25428855 Free PMC article. Review.
-
Reversed electrogenic sodium bicarbonate cotransporter 1 is the major acid loader during recovery from cytosolic alkalosis in mouse cortical astrocytes.J Physiol. 2015 Aug 15;593(16):3533-47. doi: 10.1113/JP270086. Epub 2015 Jun 25. J Physiol. 2015. PMID: 25990710 Free PMC article.
-
Intracellular pH Regulation in Ventricular Myocytes: Implications for Cardiac Health and Disease.Circ Res. 2025 Jun 6;136(12):1636-1656. doi: 10.1161/CIRCRESAHA.125.325386. Epub 2025 Jun 5. Circ Res. 2025. PMID: 40472057 Review.
Cited by
-
Role of SLC4 and SLC26 solute carriers during oxidative stress.Acta Physiol (Oxf). 2022 May;235(1):e13796. doi: 10.1111/apha.13796. Epub 2022 Mar 1. Acta Physiol (Oxf). 2022. PMID: 35143116 Free PMC article. Review.
-
Molecular dynamics simulations of lipid-protein interactions in SLC4 proteins.Biophys J. 2024 Jun 18;123(12):1705-1721. doi: 10.1016/j.bpj.2024.05.013. Epub 2024 May 17. Biophys J. 2024. PMID: 38760929 Free PMC article.
-
Potential Theranostic Roles of SLC4 Molecules in Human Diseases.Int J Mol Sci. 2023 Oct 13;24(20):15166. doi: 10.3390/ijms242015166. Int J Mol Sci. 2023. PMID: 37894847 Free PMC article. Review.
-
Bicarbonate ion transport by the electrogenic Na+ /HCO3- cotransporter, NBCe1, is required for normal electrical slow-wave activity in mouse small intestine.Neurogastroenterol Motil. 2021 Sep;33(9):e14149. doi: 10.1111/nmo.14149. Epub 2021 Apr 10. Neurogastroenterol Motil. 2021. PMID: 33837991 Free PMC article.
-
Beat-to-beat dynamic regulation of intracellular pH in cardiomyocytes.iScience. 2021 Dec 13;25(1):103624. doi: 10.1016/j.isci.2021.103624. eCollection 2022 Jan 21. iScience. 2021. PMID: 35005560 Free PMC article.
References
-
- Vaughan-Jones RD, Spitzer KW. Role of bicarbonate in the regulation of intracellular pH in the mammalian ventricular myocyte. Biochem Cell Biol. 2002;80:579–596. - PubMed
-
- Casey JR. Why bicarbonate? Biochem Cell Biol. 2006;84:930–939. - PubMed
-
- Karmazyn M, Kilić A, Javadov S. The role of NHE-1 in myocardial hypertrophy and remodelling. J Mol Cell Cardiol. 2008;44:647–653. - PubMed
-
- Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–331. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

