GH Dysfunction in Engrailed-2 Knockout Mice, a Model for Autism Spectrum Disorders
- PMID: 25225635
- PMCID: PMC4150208
- DOI: 10.3389/fped.2014.00092
GH Dysfunction in Engrailed-2 Knockout Mice, a Model for Autism Spectrum Disorders
Abstract
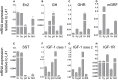
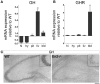
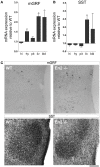
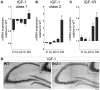
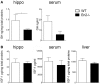
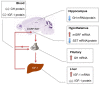
Insulin-like growth factor 1 (IGF-1) signaling promotes brain development and plasticity. Altered IGF-1 expression has been associated to autism spectrum disorders (ASD). IGF-1 levels were found increased in the blood and decreased in the cerebrospinal fluid of ASD children. Accordingly, IGF-1 treatment can rescue behavioral deficits in mouse models of ASD, and IGF-1 trials have been proposed for ASD children. IGF-1 is mainly synthesized in the liver, and its synthesis is dependent on growth hormone (GH) produced in the pituitary gland. GH also modulates cognitive functions, and altered levels of GH have been detected in ASD patients. Here, we analyzed the expression of GH, IGF-1, their receptors, and regulatory hormones in the neuroendocrine system of adult male mice lacking the homeobox transcription factor Engrailed-2 (En2 (-/-) mice). En2 (-/-) mice display ASD-like behaviors (social interactions, defective spatial learning, increased seizure susceptibility) accompanied by relevant neuropathological changes (loss of cerebellar and forebrain inhibitory neurons). Recent studies showed that En2 modulates IGF-1 activity during postnatal cerebellar development. We found that GH mRNA expression was markedly deregulated throughout the neuroendocrine axis in En2 (-/-) mice, as compared to wild-type controls. In mutant mice, GH mRNA levels were significantly increased in the pituitary gland, blood, and liver, whereas decreased levels were detected in the hippocampus. These changes were paralleled by decreased levels of GH protein in the hippocampus but not other tissues of En2 (-/-) mice. IGF-1 mRNA was significantly up-regulated in the liver and down-regulated in the En2 (-/-) hippocampus, but no differences were detected in the levels of IGF-1 protein between the two genotypes. Our data strengthen the notion that altered GH levels in the hippocampus may be involved in learning disabilities associated to ASD.
Keywords: autism spectrum disorders; growth hormone; hippocampus; insulin-like growth factor; liver; mouse model; neuroendocrine axis; pituitary gland.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

