Fatty Acid-Derived Biofuels and Chemicals Production in Saccharomyces cerevisiae
- PMID: 25225637
- PMCID: PMC4150446
- DOI: 10.3389/fbioe.2014.00032
Fatty Acid-Derived Biofuels and Chemicals Production in Saccharomyces cerevisiae
Abstract
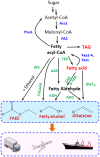
Volatile energy costs and environmental concerns have spurred interest in the development of alternative, renewable, sustainable, and cost-effective energy resources. Environment-friendly processes involving microbes can be used to synthesize advanced biofuels. These fuels have the potential to replace fossil fuels in supporting high-power demanding machinery such as aircrafts and trucks. From an engineering perspective, the pathway for fatty acid biosynthesis is an attractive route for the production of advanced fuels such as fatty acid ethyl esters, fatty alcohols, and alkanes. The robustness and excellent accessibility to molecular genetics make the yeast Saccharomyces cerevisiae a suitable host for the purpose of bio-manufacturing. Recent advances in metabolic engineering, as well as systems and synthetic biology, have now provided the opportunity to engineer yeast metabolism for the production of fatty acid-derived fuels and chemicals.
Keywords: alkanes/alkene; fatty acid ethyl ester; fatty acid metabolism; fatty alcohol; yeast.
Figures

References
-
- Bernard A., Domergue F., Pascal S., Jetter R., Renne C., Faure J. D., et al. (2012). Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 24, 3106–311810.1105/tpc.112.099796 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

