Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux
- PMID: 25225670
- PMCID: PMC4185247
- DOI: 10.4049/jimmunol.1400582
Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux
Abstract
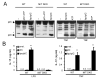
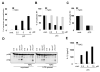
The nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3 (Nlrp3) inflammasome plays an important role in inflammation by controlling the maturation and secretion of the cytokines IL-1β and IL-18 in response to multiple stimuli including pore-forming toxins, particulate matter, and ATP. Although the pathways activated by the latter stimuli lead to a decrease in intracellular K(+) concentration, which is required for inflammasome activation, the mechanism by which microbial RNA activates Nlrp3, remains poorly understood. In this study, we found that cytosolic poly(I:C), but not total RNA from healthy macrophages, macrophages undergoing pyroptosis, or mitochondrial RNA, induces caspase-1 activation and IL-1β release through the Nlrp3 inflammasome. Experiments with macrophages deficient in Tlr3, Myd88, or Trif, indicate that poly(I:C) induces Nlrp3 activation independently of TLR signaling. Further analyses revealed that the cytosolic sensors Rig-I and melanoma differentiation-associated gene 5 act redundantly via the common adaptor mitochondrial antiviral signaling (Mavs) to induce Nlrp3 activation in response to poly(I:C), but not ATP or nigericin. Mechanistically, Mavs triggered membrane permeabilization and K(+) efflux independently of the inflammasome which were required for poly(I:C)-induced Nlrp3 activation. We conclude that poly (I:C) activates the inflammasome through an Mavs-dependent surveillance pathway that converges into a common K(+) lowering step in the cytosol that is essential for the induction of Nlrp3 activation.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

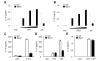
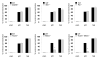

Similar articles
-
The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome.Immunity. 2013 Jul 25;39(1):123-35. doi: 10.1016/j.immuni.2013.07.001. Epub 2013 Jul 18. Immunity. 2013. PMID: 23871209 Free PMC article.
-
Suppression of ribosomal function triggers innate immune signaling through activation of the NLRP3 inflammasome.PLoS One. 2012;7(5):e36044. doi: 10.1371/journal.pone.0036044. Epub 2012 May 14. PLoS One. 2012. PMID: 22606244 Free PMC article.
-
Cytosolic antiviral RNA recognition pathway activates caspases 1 and 3.J Immunol. 2008 Feb 1;180(3):1749-57. doi: 10.4049/jimmunol.180.3.1749. J Immunol. 2008. PMID: 18209072
-
Mechanism and Regulation of NLRP3 Inflammasome Activation.Trends Biochem Sci. 2016 Dec;41(12):1012-1021. doi: 10.1016/j.tibs.2016.09.002. Epub 2016 Sep 23. Trends Biochem Sci. 2016. PMID: 27669650 Free PMC article. Review.
-
Toll-like receptor, RIG-I-like receptors and the NLRP3 inflammasome: key modulators of innate immune responses to double-stranded RNA viruses.Cytokine Growth Factor Rev. 2011 Apr;22(2):63-72. doi: 10.1016/j.cytogfr.2011.02.001. Epub 2011 Apr 3. Cytokine Growth Factor Rev. 2011. PMID: 21466970 Free PMC article. Review.
Cited by
-
Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3.Nat Commun. 2015 Jun 24;6:7515. doi: 10.1038/ncomms8515. Nat Commun. 2015. PMID: 26104484 Free PMC article.
-
Initiation and perpetuation of NLRP3 inflammasome activation and assembly.Immunol Rev. 2015 May;265(1):35-52. doi: 10.1111/imr.12286. Immunol Rev. 2015. PMID: 25879282 Free PMC article. Review.
-
NLRP3 Inflammasome in the Pathophysiology of Hemorrhagic Stroke: A Review.Curr Neuropharmacol. 2019;17(7):582-589. doi: 10.2174/1570159X17666181227170053. Curr Neuropharmacol. 2019. PMID: 30592254 Free PMC article. Review.
-
The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation.Int J Mol Sci. 2019 Jul 6;20(13):3328. doi: 10.3390/ijms20133328. Int J Mol Sci. 2019. PMID: 31284572 Free PMC article. Review.
-
Targeting intrinsic RIG-I signaling turns melanoma cells into type I interferon-releasing cellular antitumor vaccines.Oncoimmunology. 2019 Feb 11;8(4):e1570779. doi: 10.1080/2162402X.2019.1570779. eCollection 2019. Oncoimmunology. 2019. PMID: 30906666 Free PMC article.
References
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. - PubMed
-
- Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. - PubMed
-
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual review of immunology. 2009;27:229–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

