Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications
- PMID: 25226290
- PMCID: PMC4297889
- DOI: 10.1210/jc.2014-1203
Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications
Abstract
Context: Reliable thyroglobulin (Tg) autoantibody (TgAb) detection before Tg testing for differentiated thyroid cancer (DTC) is critical when TgAb status (positive/negative) is used to authenticate sensitive second-generation immunometric assay ((2G)IMA) measurements as free from TgAb interference and when reflexing "TgAb-positive" sera to TgAb-resistant, but less sensitive, Tg methodologies (radioimmunoassay [RIA] or liquid chromatography-tandem mass spectrometry [LC-MS/MS]).
Objective: The purpose of this study was to assess how different Kronus (K) vs Roche (R) TgAb method cutoffs for "positivity" influence false-negative vs false-positive serum TgAb misclassifications that may reduce the clinical utility of reflex Tg testing.
Methods: Serum Tg(2G)IMA, TgRIA, and TgLC-MS/MS measurements for 52 TgAb-positive and 37 TgAb-negative patients with persistent/recurrent DTC were compared. A total of 1426 DTC sera with TgRIA of ≥ 1.0 μg/L had false-negative and false-positive TgAb frequencies determined using low Tg(2G)IMA/TgRIA ratios (<75%) to indicate TgAb interference.
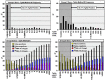
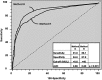
Results: TgAb-negative patients with disease displayed Tg(2G)IMA, TgRIA, and TgLC-MS/MS serum discordances (% coefficient of variation = 24 ± 20%, range, 0%-100%). Of the TgAb-positive patients with disease, 98% had undetectable/lower Tg(2G)IMA vs either TgRIA or TgLC-MS/MS (P < .01), whereas 8 of 52 (15%) had undetectable Tg(2G)IMA + TgLC-MS/MS associated with TgRIA of ≥ 1.0 μg/L. Receiver operating characteristic curve analysis reported more sensitivity for TgAb method K vs R (81.9% vs 69.1%, P < .001), but receiver operating characteristic curve cutoffs (>0.6 kIU/L [K] vs >40 kIU/L [R]) had unacceptably high false-negative frequencies (22%-32%), whereas false positives approximated 12%. Functional sensitivity cutoffs minimized false negatives (13.5% [K] vs 21.3% [R], P < .01) and severe interferences (Tg(2G)IMA, <0.10 μg/L) (0.7% [K] vs 2.4% [R], P < .05) but false positives approximated 23%.
Conclusions: Reliable detection of interfering TgAbs is method and cutoff dependent. No cutoff eliminated both false-negative and false-positive TgAb misclassifications. Functional sensitivity cutoffs were optimal for minimizing false negatives but have inherent imprecision (20% coefficient of variation) that, exacerbated by TgAb biologic variability during DTC monitoring, could cause TgAb status to fluctuate for patients with low TgAb concentrations, prompting unnecessary Tg method changes and disrupting Tg monitoring. Laboratories using reflexing should limit Tg method changes by considering a patient's Tg + TgAb testing history in addition to current TgAb status before Tg method selection.
Figures


References
-
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. - PubMed
-
- Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127. - PubMed
-
- Görges R, Maniecki M, Jentzen W, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153:49–55. - PubMed
-
- Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods—strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013;27:701–712. - PubMed
-
- Verburg FA, Luster M, Cupini C, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23:1211–1225. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

