Bioluminescent imaging of genetically selected induced pluripotent stem cell-derived cardiomyocytes after transplantation into infarcted heart of syngeneic recipients
- PMID: 25226590
- PMCID: PMC4167328
- DOI: 10.1371/journal.pone.0107363
Bioluminescent imaging of genetically selected induced pluripotent stem cell-derived cardiomyocytes after transplantation into infarcted heart of syngeneic recipients
Abstract
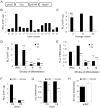
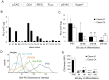
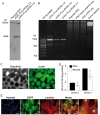
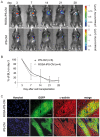
Cell loss after transplantation is a major limitation for cell replacement approaches in regenerative medicine. To assess the survival kinetics of induced pluripotent stem cell (iPSC)-derived cardiomyocytes (CM) we generated transgenic murine iPSC lines which, in addition to CM-specific expression of puromycin N-acetyl-transferase and enhanced green fluorescent protein (EGFP), also constitutively express firefly luciferase (FLuc) for bioluminescence (BL) in vivo imaging. While undifferentiated iPSC lines generated by random integration of the transgene into the genome retained stable FLuc activity over many passages, the BL signal intensity was strongly decreased in purified iPS-CM compared to undifferentiated iPSC. Targeted integration of FLuc-expression cassette into the ROSA26 genomic locus using zinc finger nuclease (ZFN) technology strongly reduced transgene silencing in iPS-CM, leading to a several-fold higher BL compared to iPS-CM expressing FLuc from random genomic loci. To investigate the survival kinetics of iPS-CM in vivo, purified CM obtained from iPSC lines expressing FLuc from a random or the ROSA26 locus were transplanted into cryoinfarcted hearts of syngeneic mice. Engraftment of viable cells was monitored by BL imaging over 4 weeks. Transplanted iPS-CM were poorly retained in the myocardium independently of the cell line used. However, up to 8% of cells survived for 28 days at the site of injection, which was confirmed by immunohistological detection of EGFP-positive iPS-CM in the host tissue. Transplantation of iPS-CM did not affect the scar formation or capillary density in the periinfarct region of host myocardium. This report is the first to determine the survival kinetics of drug-selected iPS-CM in the infarcted heart using BL imaging and demonstrates that transgene silencing in the course of iPSC differentiation can be greatly reduced by employing genome editing technology. FLuc-expressing iPS-CM generated in this study will enable further studies to reduce their loss, increase long-term survival and functional integration upon transplantation.
Conflict of interest statement
Figures

References
-
- Burt RK, Chen YH, Verda L, Lucena C, Navale S, et al. (2012) Mitotically inactivated embryonic stem cells can be used as an in vivo feeder layer to nurse damaged myocardium after acute myocardial infarction: a preclinical study. Circ Res 111: 1286–1296. - PubMed
-
- Singla DK, Lyons GE, Kamp TJ (2007) Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am J Physiol Heart Circ Physiol 293: H1308–1314. - PubMed
-
- Yan B, Abdelli LS, Singla DK (2011) Transplanted induced pluripotent stem cells improve cardiac function and induce neovascularization in the infarcted hearts of db/db mice. Mol Pharm 8: 1602–1610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

