Volumetric assessment of tumour response using functional MR imaging in patients with hepatocellular carcinoma treated with a combination of doxorubicin-eluting beads and sorafenib
- PMID: 25226843
- PMCID: PMC4324620
- DOI: 10.1007/s00330-014-3412-6
Volumetric assessment of tumour response using functional MR imaging in patients with hepatocellular carcinoma treated with a combination of doxorubicin-eluting beads and sorafenib
Abstract
Objective: To prospectively assess treatment response using volumetric functional magnetic resonance imaging (MRI) metrics in patients with hepatocellular carcinoma (HCC) treated with the combination of doxorubicin-eluting bead-transarterial chemoembolization (DEB TACE) and sorafenib.
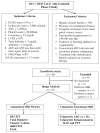
Methods: A single center study enrolled 41 patients treated with systemic sorafenib, 400 mg twice a day, combined with DEB TACE. All patients had a pre-treatment and 3-4 week post-treatment MRI. Anatomic response criteria (RECIST, mRECIST and EASL) and volumetric functional response (ADC, enhancement) were assessed. Statistical analyses included paired Student's t-test, Kaplan-Meier curves, Cohen's Kappa, and multivariate cox proportional hazard model.
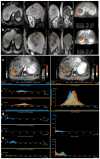
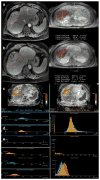
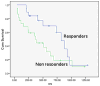
Results: Median tumour size by RECIST remained unchanged post-treatment (8.3 ± 4.1 cm vs. 8.1 ± 4.3 cm, p = 0.44). There was no significant survival difference for early response by RECIST (p = 0.93). EASL and mRECIST could not be analyzed in 12 patients. Volumetric ADC increased significantly (1.32 × 10(-3) mm(2)/sec to 1.60 × 10(-3) mm(2)/sec, p < 0.001), and volumetric enhancement decreased significantly in HAP (38.2% to 17.6%, p < 0.001) and PVP (76.6% to 41.2%, p < 0.005). Patients who demonstrated ≥ 65% decrease PVP enhancement had significantly improved overall survival compared to non-responders (p < 0.005).
Conclusion: Volumetric PVP enhancement was demonstrated to be significantly correlated with survival in the combination of DEB TACE and sorafenib for patients with HCC, enabling precise stratification of responders and non-responders.
Key points: • PVP enhancement is significantly correlated with survival in responders (p < 0.005). • There was no significant survival difference for early response using RECIST (p = 0.93). • mRECIST or EASL could not assess tumour response in 29% of patients.
Figures

References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. - PubMed
-
- Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. - PubMed
-
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

