Influence of early goal-directed therapy using arterial waveform analysis on major complications after high-risk abdominal surgery: study protocol for a multicenter randomized controlled superiority trial
- PMID: 25227114
- PMCID: PMC4175278
- DOI: 10.1186/1745-6215-15-360
Influence of early goal-directed therapy using arterial waveform analysis on major complications after high-risk abdominal surgery: study protocol for a multicenter randomized controlled superiority trial
Abstract
Background: Early goal-directed therapy refers to the use of predefined hemodynamic goals to optimize tissue oxygen delivery in critically ill patients. Its application in high-risk abdominal surgery is, however, hindered by safety concerns and practical limitations of perioperative hemodynamic monitoring. Arterial waveform analysis provides an easy, minimally invasive alternative to conventional monitoring techniques, and could be valuable in early goal-directed strategies. We therefore investigate the effects of early goal-directed therapy using arterial waveform analysis on complications, quality of life and healthcare costs after high-risk abdominal surgery.
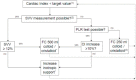
Methods/design: In this multicenter, randomized, controlled superiority trial, 542 patients scheduled for elective, high-risk abdominal surgery will be included. Patients are allocated to standard care (control group) or early goal-directed therapy (intervention group) using a randomization procedure stratified by center and type of surgery. In the control group, standard perioperative hemodynamic monitoring is applied. In the intervention group, early goal-directed therapy is added to standard care, based on continuous monitoring of cardiac output with arterial waveform analysis. A treatment algorithm is used as guidance for fluid and inotropic therapy to maintain cardiac output above a preset, age-dependent target value. The primary outcome measure is a combined endpoint of major complications in the first 30 days after the operation, including mortality. Secondary endpoints are length of stay in the hospital, length of stay in the intensive care or post-anesthesia care unit, the number of minor complications, quality of life, cost-effectiveness and one-year mortality and morbidity.
Discussion: Before the start of the study, hemodynamic optimization by early goal-directed therapy with arterial waveform analysis had only been investigated in small, single-center studies, including minor complications as primary endpoint. Moreover, these studies did not include quality of life, healthcare costs, and long-term outcome in their analysis. As a result, the definitive role of arterial waveform analysis in the perioperative hemodynamic assessment and care for high-risk surgical patients is unknown, which gave rise to the present trial. Patient inclusion started in May 2012 and is expected to end in 2016.
Trial registration: This trial was registered in the Dutch Trial Register (registration number NTR3380) on 3 April 2012.
Figures

Similar articles
-
Perioperative goal-directed therapy in high-risk abdominal surgery. A multicenter randomized controlled superiority trial.J Clin Anesth. 2021 Dec;75:110506. doi: 10.1016/j.jclinane.2021.110506. Epub 2021 Sep 15. J Clin Anesth. 2021. PMID: 34536718 Clinical Trial.
-
Goal-Directed Fluid Therapy Guided by Cardiac Monitoring During High-Risk Abdominal Surgery in Adult Patients: Cost-Effectiveness Analysis of Esophageal Doppler and Arterial Pulse Pressure Waveform Analysis.Value Health. 2015 Jul;18(5):605-13. doi: 10.1016/j.jval.2015.04.005. Epub 2015 Jun 10. Value Health. 2015. PMID: 26297088
-
Individualized, perioperative, hemodynamic goal-directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial.Trials. 2018 May 9;19(1):273. doi: 10.1186/s13063-018-2620-9. Trials. 2018. PMID: 29743101 Free PMC article.
-
Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review.JAMA. 2014 Jun 4;311(21):2181-90. doi: 10.1001/jama.2014.5305. JAMA. 2014. PMID: 24842135 Clinical Trial.
-
Impact of hemodynamic goal-directed resuscitation on mortality in adult critically ill patients: a systematic review and meta-analysis.J Clin Monit Comput. 2018 Jun;32(3):403-414. doi: 10.1007/s10877-017-0032-0. Epub 2017 Jun 8. J Clin Monit Comput. 2018. PMID: 28593456 Free PMC article.
Cited by
-
Stroke volume variation to guide fluid therapy: is it suitable for high-risk surgical patients? A terminated randomized controlled trial.Perioper Med (Lond). 2015 Jul 22;4:6. doi: 10.1186/s13741-015-0016-x. eCollection 2015. Perioper Med (Lond). 2015. PMID: 26203353 Free PMC article.
-
Effect of goal-directed haemodynamic therapy guided by non-invasive monitoring on perioperative complications in elderly hip fracture patients within an enhanced recovery pathway.Perioper Med (Lond). 2022 Aug 10;11(1):46. doi: 10.1186/s13741-022-00277-w. Perioper Med (Lond). 2022. PMID: 35945605 Free PMC article.
-
Training nurses in a competency framework to support adults with epilepsy and intellectual disability: the EpAID cluster RCT.Health Technol Assess. 2018 Feb;22(10):1-104. doi: 10.3310/hta22100. Health Technol Assess. 2018. PMID: 29457585 Free PMC article. Clinical Trial.
-
Can perioperative pCO2 gaps predict complications in patients undergoing major elective abdominal surgery randomized to goal-directed therapy or standard care? A secondary analysis.J Clin Monit Comput. 2024 Apr;38(2):469-477. doi: 10.1007/s10877-023-01117-y. Epub 2024 Jan 22. J Clin Monit Comput. 2024. PMID: 38252193 Free PMC article. Clinical Trial.
-
Improving outcomes in adults with epilepsy and intellectual disability (EpAID) using a nurse-led intervention: study protocol for a cluster randomised controlled trial.Trials. 2016 Jun 24;17(1):297. doi: 10.1186/s13063-016-1429-7. Trials. 2016. PMID: 27342377 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

