Erythropoietin rs1617640 G allele associates with an attenuated rise of serum erythropoietin and a marked decline of hemoglobin in hepatitis C patients undergoing antiviral therapy
- PMID: 25227310
- PMCID: PMC4175618
- DOI: 10.1186/1471-2334-14-503
Erythropoietin rs1617640 G allele associates with an attenuated rise of serum erythropoietin and a marked decline of hemoglobin in hepatitis C patients undergoing antiviral therapy
Abstract
Background: A decline in hemoglobin (Hb) concentration during antiviral therapy in chronic hepatitis C (CHC) is a serious side effect. It may compel to dose reduction or even termination of antiviral treatment. The activation of erythropoietin (EPO) synthesis as a physiological response to anemia and its relation to a genetic variation within the EPO gene has not been evaluated yet.
Methods: Data of 348 CHC patients were reviewed retrospectively. Samples were genotyped for EPO rs1617640 and inosine triphosphatase (ITPA) rs1127354. Serum EPO concentrations were determined before and during therapy. Primary endpoints were set as Hb decline >3 g/dl at weeks 4 and 12.
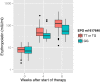
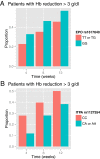
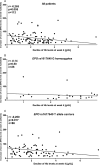
Results: EPO rs1617640 G homozygotes showed a significantly lower rise of serum EPO level over time than T allele carriers (p < 0.001). The cumulative frequency of a significant Hb reduction added up to 40%. Multivariate analysis revealed that besides age, ribavirin starting dose and baseline Hb also EPO rs1617640 G homozygosity associates with Hb reduction at week 4 (p = 0.025) and 12 (p = 0.029), while ITPA C homozygotes are at risk for Hb decline particularly early during treatment. Furthermore, EPO rs1617640 G homozygotes were more frequently in need for blood transfusion, epoetin-α supplementation, or ribavirin dose reduction (p < 0.001).
Conclusions: Our data suggest that EPO rs1617640 genotype, the rise of serum EPO concentration as well as ITPA rs1127354 genotype are promising parameters to evaluate the Hb decline during antiviral therapy. A rational adjustment of therapy with epoetin-α supplementation might prevent serious adverse events or the need to terminate treatment.
Figures
Similar articles
-
Impact of ITPA gene polymorphism for predicting anemia and treatment outcome in HCV infected patients taking Sofosbuvir Ribavirin therapy.BMC Infect Dis. 2024 Mar 11;24(1):301. doi: 10.1186/s12879-024-09188-1. BMC Infect Dis. 2024. PMID: 38468199 Free PMC article.
-
Polymorphism of the inosine triphosphate pyrophosphatase gene predicts ribavirin-induced anemia in chronic hepatitis C patients.Mol Med Rep. 2012 Feb;5(2):517-20. doi: 10.3892/mmr.2011.659. Epub 2011 Nov 2. Mol Med Rep. 2012. PMID: 22052220
-
ITPA gene polymorphism (94C>A) effects on ribavirin-induced anemia during therapy in Egyptian patients with chronic hepatitis C.J Med Virol. 2017 Oct;89(10):1823-1829. doi: 10.1002/jmv.24844. Epub 2017 May 29. J Med Virol. 2017. PMID: 28480960
-
Anemia and thrombocytosis induced by ribavirin monotherapy in patients with chronic hepatitis C.J Gastroenterol. 2012 Nov;47(11):1228-37. doi: 10.1007/s00535-012-0579-y. Epub 2012 Mar 30. J Gastroenterol. 2012. PMID: 22460221 Clinical Trial.
-
ITPA deficiency and ribavirin level are still predictive of anaemia in HCV-HIV-coinfected patients receiving ribavirin combined with a first-generation DAA (ANRS HC27 study).Antivir Ther. 2017;22(6):461-469. doi: 10.3851/IMP3074. Epub 2016 Sep 1. Antivir Ther. 2017. PMID: 27583701
Cited by
-
The Erythropoetin rs1617640 Gene Polymorphism Associates with Hemoglobin Levels, Hematocrit and Red Blood Cell Count in Patients with Peripheral Arterial Disease.Genes (Basel). 2020 Nov 4;11(11):1305. doi: 10.3390/genes11111305. Genes (Basel). 2020. PMID: 33158076 Free PMC article.
-
The Functional Erythropoetin rs1617640 Gene Polymorphism does not Affect Life Expectancy of Patients with Peripheral Arterial Disease.Rev Cardiovasc Med. 2023 Jul 13;24(7):199. doi: 10.31083/j.rcm2407199. eCollection 2023 Jul. Rev Cardiovasc Med. 2023. PMID: 39077006 Free PMC article.
-
Identification and single-base gene-editing functional validation of a cis-EPO variant as a genetic predictor for EPO-increasing therapies.Am J Hum Genet. 2022 Sep 1;109(9):1638-1652. doi: 10.1016/j.ajhg.2022.08.004. Am J Hum Genet. 2022. PMID: 36055212 Free PMC article.
References
-
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS, IDEAL Study Team Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. - DOI - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. - DOI - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. - DOI - PubMed
-
- McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J, Bsharat M, George S, Kauffman RS, Adda N, Di Bisceglie AM, PROVE3 Study Team Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. - DOI - PubMed
-
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R, HCV RESPOND-2 Investigators Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/14/503/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

