The autoinhibitory C-terminal SH2 domain of phospholipase C-γ2 stabilizes B cell receptor signalosome assembly
- PMID: 25227611
- PMCID: PMC4180509
- DOI: 10.1126/scisignal.2005392
The autoinhibitory C-terminal SH2 domain of phospholipase C-γ2 stabilizes B cell receptor signalosome assembly
Abstract
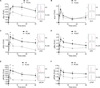
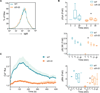
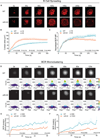
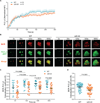
The binding of antigen to the B cell receptor (BCR) stimulates the assembly of a signaling complex (signalosome) composed initially of the kinases Lyn, spleen tyrosine kinase (Syk), and Bruton's tyrosine kinase (Btk), as well as the adaptor protein B cell linker (BLNK). Together, these proteins recruit and activate phospholipase C-γ2 (PLC-γ2), a critical effector that stimulates increases in intracellular Ca(2+) and activates various signaling pathways downstream of the BCR. Individuals with one copy of a mutant PLCG2 gene, which encodes a variant PLC-γ2 that lacks the autoinhibitory C-terminal Src homology 2 (cSH2) domain, exhibit PLC-γ2-associated antibody deficiency and immune dysregulation (PLAID). Paradoxically, although COS-7 cells expressing the variant PLC-γ2 show enhanced basal and stimulated PLC-γ2 activity, B cells from PLAID patients show defective intracellular Ca(2+) responses upon cross-linking of the BCR. We found that the cSH2 domain of PLC-γ2 played a critical role in stabilizing the early signaling complex that is stimulated by BCR cross-linking. In the presence of the variant PLC-γ2, Syk, Btk, and BLNK were only weakly phosphorylated and failed to stably associate with the BCR. Thus, BCRs could not form stable clusters, resulting in dysregulation of downstream signaling and trafficking of the BCR. Thus, the cSH2 domain functions not only to inhibit the active site of PLC-γ2 but also to directly or indirectly stabilize the early BCR signaling complex.
Copyright © 2014, American Association for the Advancement of Science.
Figures




References
-
- Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev. 2009;228:132–148. - PubMed
-
- Kadamur G, Ross EM. Mammalian phospholipase C. Annual review of physiology. 2013;75:127–154. - PubMed
-
- Hilgemann DW. Local PIP(2) signals: when, where, and how? Pflugers Archiv: European journal of physiology. 2007;455:55–67. - PubMed
-
- Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP, Carpenter CL. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

