Comparison of chromosome breakage in non-mosaic and mosaic patients with Fanconi anemia, relatives, and patients with other inherited bone marrow failure syndromes
- PMID: 25227706
- PMCID: PMC4225000
- DOI: 10.1159/000366251
Comparison of chromosome breakage in non-mosaic and mosaic patients with Fanconi anemia, relatives, and patients with other inherited bone marrow failure syndromes
Abstract
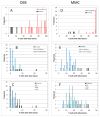
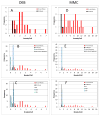
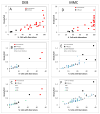
Fanconi anemia (FA) is a rare inherited bone marrow failure syndrome (IBMFS). Affected individuals must be distinguished from relatives, patients with mosaicism must be identified, and patients with other IBMFS classified as non-FA. The diagnostic feature of FA is increased chromosomal breakage in blood lymphocytes cultured with diepoxybutane or mitomycin C. Here, we sought a method to uniquely identify patients with FA with mosaicism, using cells from participants in the National Cancer Institute IBMFS cohort. Lymphocytes were treated with diepoxybutane or mitomycin C, and metaphases scored for breaks and radials. Analyses included the percentage of cells with any aberration, breaks per cell, and breaks per aberrant cell. There were 26 patients with FA (4 mosaics), 46 FA relatives, and 62 patients with a non-FA IBMFS. By all analytic methods, patients with FA were abnormal compared with other groups. Those with FA mosaicism had more breakage than relatives or patients with non-FA IBMFS, but there was some individual overlap. The choices of clastogen are laboratory-dependent, but there was no method or analysis of lymphocytes that clearly distinguished all individuals mosaic for FA from relatives or patients with other IBMFS. Thus, genotyping remains the best method for providing absolute clarity.
© 2014 S. Karger AG, Basel.
Figures




References
-
- Alter BP, Joenje H, Oostra AB, Pals G. Fanconi anemia: adult head and neck cancer and hematopoietic mosaicism. Arch Otolaryngol Head Neck Surg. 2005;131:635–639. - PubMed
-
- Auerbach AD, Adler B, Chaganti RSK. Prenatal and postnatal diagnosis and carrier detection of Fanconi anemia by a cytogenetic method. Pediatrics. 1981;67:128–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

