Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice
- PMID: 25227984
- PMCID: PMC4363301
- DOI: 10.1038/mi.2014.84
Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice
Abstract
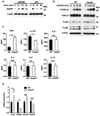
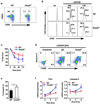
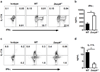
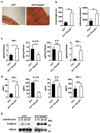
Mitogen-activated protein kinase (MAPK) phosphatases are dual-specificity phosphatases (DUSPs) that dephosphorylate phosphothreonine and phosphotyrosine residues within MAPKs. DUSP6 preferentially dephosphorylates extracellular signal-regulated kinases 1 and 2 (ERK1/2) rendering them inactive. Here, we study the role of DUSP6 in CD4(+) T-cell function, differentiation, and inflammatory profile in the colon. Upon T-cell receptor (TCR) stimulation, DUSP6 knockout (Dusp6(-/-)) CD4(+) T cells showed increased ERK1/2 activation, proliferation, T helper 1 differentiation, and interferon-γ production, as well as a marked decrease in survival, interleukin- 17A (IL-17A) secretion, and regulatory T-cell function. To analyze the role of DUSP6 in vivo, we employed the Il10(-/-) model of colitis and generated Il10(-/-)/Dusp6(-/-) double-knockout mice. Il10(-/-)/Dusp6(-/-) mice suffered from accelerated and exacerbated spontaneous colitis, which was prevented by ERK1/2 inhibition. ERK1/2 inhibition also augmented regulatory T-cell differentiation in vitro and in vivo in both C57Bl/6 and Dusp6(-/-) mice. In summary, DUSP6 regulates CD4(+) T-cell activation and differentiation by inhibiting the TCR-dependent ERK1/2 activation. DUSP6 might therefore be a potential intervention target for limiting aberrant T-cell responses in T-cell-mediated diseases, such as inflammatory bowel disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zassadowski F, Rochette-Egly C, Chomienne C, Cassinat B. Regulation of the transcriptional activity of nuclear receptors by the MEK/ERK1/2 pathway. Cell Signal. 2012;24(12):2369–2377. - PubMed
-
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12(2):186–192. - PubMed
-
- Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, et al. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271(44):27205–27208. - PubMed
-
- Stewart AE, Dowd S, Keyse SM, McDonald NQ. Crystal structure of the MAPK phosphatase Pyst1 catalytic domain and implications for regulated activation. Nat Struct Biol. 1999;6(2):174–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

