Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites
- PMID: 25227985
- PMCID: PMC4363306
- DOI: 10.1038/mi.2014.85
Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites
Abstract
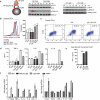
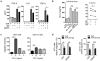
Antigen-mediated cross-linking of Immunoglobulin E (IgE) bound to mast cells/basophils via FcɛRI, the high affinity IgE Fc-receptor, is a well-known trigger of allergy. In humans, but not mice, dendritic cells (DCs) also express FcɛRI that is constitutively occupied with IgE. In contrast to mast cells/basophils, the consequences of IgE/FcɛRI signals for DC function remain poorly understood. We show that humanized mice that express FcɛRI on DCs carry IgE like non-allergic humans and do not develop spontaneous allergies. Antigen-specific IgE/FcɛRI cross-linking fails to induce maturation or production of inflammatory mediators in human DCs and FcɛRI-humanized DCs. Furthermore, conferring expression of FcɛRI to DCs decreases the severity of food allergy and asthma in disease-relevant models suggesting anti-inflammatory IgE/FcɛRI signals. Consistent with the improved clinical parameters in vivo, antigen-specific IgE/FcɛRI cross-linking on papain or lipopolysaccharide-stimulated DCs inhibits the production of pro-inflammatory cytokines and chemokines. Migration assays confirm that the IgE-dependent decrease in cytokine production results in diminished recruitment of mast cell progenitors; providing a mechanistic explanation for the reduced mast cell-dependent allergic phenotype observed in FcɛRI-humanized mice. Our study demonstrates a novel immune regulatory function of IgE and proposes that DC-intrinsic IgE signals serve as a feedback mechanism to restrain allergic tissue inflammation.
Figures


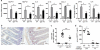

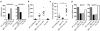

References
-
- Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: a major global health concern. Current opinion in allergy and clinical immunology. 2012;12:39–41. - PubMed
-
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature reviews. Immunology. 2008;8:205–217. - PubMed
-
- Kinet JP. The essential role of mast cells in orchestrating inflammation. Immunological reviews. 2007;217:5–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL091957/HL/NHLBI NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- R01 AI075037/AI/NIAID NIH HHS/United States
- K01DK093597/DK/NIDDK NIH HHS/United States
- R37 GM053950/GM/NIGMS NIH HHS/United States
- R01HL091957/HL/NHLBI NIH HHS/United States
- AI075037/AI/NIAID NIH HHS/United States
- R56 AI075037/AI/NIAID NIH HHS/United States
- R37GM053950/GM/NIGMS NIH HHS/United States
- CAPMC/ CIHR/Canada
- P30DK034854/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- DK53056/DK/NIDDK NIH HHS/United States
- R56 DK053056/DK/NIDDK NIH HHS/United States
- K01 DK093597/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

