The role of double-strand break repair pathways at functional and dysfunctional telomeres
- PMID: 25228584
- PMCID: PMC4292156
- DOI: 10.1101/cshperspect.a016576
The role of double-strand break repair pathways at functional and dysfunctional telomeres
Abstract
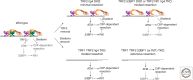
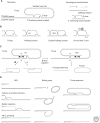
Telomeres have evolved to protect the ends of linear chromosomes from the myriad of threats posed by the cellular DNA damage signaling and repair pathways. Mammalian telomeres have to block nonhomologous end joining (NHEJ), thus preventing chromosome fusions; they need to control homologous recombination (HR), which could change telomere lengths; they have to avoid activating the ATM (ataxia telangiectasia mutated) and ATR (ATM- and RAD3-related) kinase pathways, which could induce cell cycle arrest; and they have to protect chromosome ends from hyperresection. Recent studies of telomeres have provided insights into the mechanisms of NHEJ and HR, how these double-strand break (DSB) repair pathways can be thwarted, and how telomeres have co-opted DNA repair factors to help in the protection of chromosome ends. These aspects of telomere biology are reviewed here with particular emphasis on recombination, the main focus of this collection.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Amiard S, Doudeau M, Pinte S, Poulet A, Lenain C, Faivre-Moskalenko C, Angelov D, Hug N, Vindigni A, Bouvet P, et al. 2007. A topological mechanism for TRF2-enhanced strand invasion. Nat Struct Mol Biol 14: 147–154. - PubMed
-
- Bechter OE, Shay JW, Wright WE 2004. The frequency of homologous recombination in human ALT cells. Cell Cycle 3: 547–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
