Acute sterile endophthalmitis following intravitreal bevacizumab: case series
- PMID: 25228797
- PMCID: PMC4164286
- DOI: 10.2147/OPTH.S66230
Acute sterile endophthalmitis following intravitreal bevacizumab: case series
Abstract
Background: Since the ophthalmological community adopted the use of intravitreal bevacizumab as an accepted off-label treatment for neovascular diseases, the amount of knowledge regarding its effects and properties has been increasing continually. In the last few years, there have been an increasing number of reports about sterile intraocular inflammation and intraocular pressure elevations after intravitreal bevacizumab. In the following case series, we describe the clinical presentation and outcomes of ten consecutive cases of patients developing mild-to-severe sterile intraocular inflammation after intravitreal bevacizumab and their management.
Methods: This report presents a retrospective case series. We reviewed the medical records of ten consecutive patients from a group of 46, in whom repackaged bevacizumab in individual aliquots from two vials from the same batch were used. All surgical procedures were performed using standard sterile techniques in the operating room. At each follow-up visit, patients underwent a complete ophthalmological examination including visual acuity assessment, intraocular pressure, biomicroscopy, and posterior fundus examination.
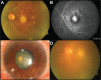
Results: Ten patients presented sterile endophthalmitis with an onset time of 3.5±1.95 days. The clinical characteristics were mild pain, slight visual loss, conjunctival hyperemia, and various degrees of intraocular inflammation with microhypopyon. All cultures were negative. All patients were managed with topical steroids and antibiotics, except two, in whom, due to severe vitreous cells, intravitreal antibiotics were used. Three patients showed a transient elevation of intraocular pressure. Only 50% of the patients regained a visual acuity equal or better to the baseline visual acuity on file.
Conclusion: The increasing number of intravitreal injections of bevacizumab applied every day, due to its widespread acceptance, might be one reason why the number of cases of sterile endophthalmitis is rising. Fast recognition and accurate differential diagnosis is important to avoid unnecessary treatments and long-term complications. The low incidence of this event should not preclude the use of intravitreal injections in eyes that could benefit greatly from this therapy.
Keywords: bevacizumab; complications; intravitreal antibiotics; pseudoendophthalmitis; vitrectomy.
Figures

Similar articles
-
Acute Sterile Endophthalmitis Following Intravitreal Rituximab Injection in Primary Vitreoretinal Lymphoma: Case Series.Ocul Immunol Inflamm. 2024 Aug;32(6):863-868. doi: 10.1080/09273948.2023.2190802. Epub 2023 Mar 23. Ocul Immunol Inflamm. 2024. PMID: 36952513
-
Characteristics of severe intraocular inflammation following intravitreal injection of bevacizumab (Avastin).Br J Ophthalmol. 2009 Apr;93(4):457-62. doi: 10.1136/bjo.2008.138479. Epub 2008 Nov 25. Br J Ophthalmol. 2009. PMID: 19033289
-
Sterile endophthalmitis after intravitreal injection of bevacizumab obtained from a single batch.Retina. 2010 Mar;30(3):485-90. doi: 10.1097/IAE.0b013e3181bd2d51. Retina. 2010. PMID: 19952993
-
Sterile endophthalmitis after intravitreal injections.Mediators Inflamm. 2012;2012:928123. doi: 10.1155/2012/928123. Epub 2012 Aug 29. Mediators Inflamm. 2012. PMID: 22973075 Free PMC article. Review.
-
Mechanisms of sterile inflammation after intravitreal injection of antiangiogenic drugs: a narrative review.Int J Retina Vitreous. 2021 May 7;7(1):37. doi: 10.1186/s40942-021-00307-7. Int J Retina Vitreous. 2021. PMID: 33962696 Free PMC article. Review.
Cited by
-
Postprocedural Endophthalmitis or Postprocedural Intraocular Inflammation: A Diagnostic Conundrum.Case Rep Ophthalmol. 2021 May 11;12(2):418-424. doi: 10.1159/000511970. eCollection 2021 May-Aug. Case Rep Ophthalmol. 2021. PMID: 34054495 Free PMC article.
-
[Silicone oil droplets in the vitreous body after intravitreal injection].Ophthalmologe. 2022 Apr;119(4):392-394. doi: 10.1007/s00347-021-01358-9. Epub 2021 Mar 9. Ophthalmologe. 2022. PMID: 33687491 German. No abstract available.
-
Intravitreal injections as a leading cause of acute postoperative endophthalmitis-a regional survey in England.Eye (Lond). 2023 Jan;37(1):163-169. doi: 10.1038/s41433-021-01886-3. Epub 2021 Dec 23. Eye (Lond). 2023. PMID: 34949787 Free PMC article.
-
Postoperative Endophthalmitis and Toxic Anterior Segment Syndrome Prophylaxis: 2020 Update.Ann Transl Med. 2020 Nov;8(22):1548. doi: 10.21037/atm-2019-rcs-02. Ann Transl Med. 2020. PMID: 33313293 Free PMC article. Review.
-
Association of compounded bevacizumab with postinjection endophthalmitis.JAMA Ophthalmol. 2015 Oct;133(10):1159-64. doi: 10.1001/jamaophthalmol.2015.2556. JAMA Ophthalmol. 2015. PMID: 26270251 Free PMC article.
References
-
- Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Mandava N, Quiroz-Mercado H. Current knowledge and trends in age-related macular degeneration: today’s and future treatments. Retina. 2013;33(8):1487–1502. - PubMed
-
- Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Quiroz-Mercado H, Mandava N. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina. 2014;34(3):423–441. - PubMed
-
- Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246(5):779–781. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

