Effects of Flavonoid Compounds on β-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons
- PMID: 25229015
- PMCID: PMC4161760
- DOI: 10.4068/cmj.2014.50.2.45
Effects of Flavonoid Compounds on β-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons
Abstract
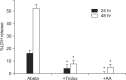
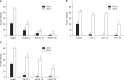
Excessive accumulation of β-amyloid peptide (Aβ) is one of the major mechanisms responsible for neuronal death in Alzheimer's disease. Flavonoids, primarily antioxidants, are a group of polyphenolic compounds synthesized in plant cells. The present study aimed to identify flavonoid compounds that could inhibit Aβ-induced neuronal death by examining the effects of various flavonoids on the neurotoxicity of Aβ fragment 25-35 (Aβ25-35) in mouse cortical cultures. Aβ25-35 induced concentration- and exposure-time-dependent neuronal death. Neuronal death induced by 20 µM Aβ25-35 was significantly inhibited by treatment with either Trolox or ascorbic acid. Among 10 flavonoid compounds tested [apigenin, baicalein, catechin, epicatechin, epigallocatechin gallate (EGCG), kaempferol, luteolin, myricetin, quercetin, and rutin], all except apigenin showed strong 1,1-diphenyl-2-pycrylhydrazyl (DPPH) scavenging activity under cell-free conditions. The flavonoid compounds except apigenin at a concentration of 30 µM also significantly inhibited neuronal death induced by 20 µM Aβ25-35 at the end of 24 hours of exposure. Epicatechin, EGCG, luteolin, and myricetin showed more potent and persistent neuroprotective action than did the other compounds. These results demonstrated that oxidative stress was involved in Aβ-induced neuronal death, and antioxidative flavonoid compounds, especially epicatechin, EGCG, luteolin, and myricetin, could inhibit neuronal death. These findings suggest that these four compounds may be developed as neuroprotective agents against Alzheimer's disease.
Keywords: Alzheimer's disease; Flavonoids; β-Amyloid peptide.
Conflict of interest statement
None declared.
Figures

References
-
- Robards K, Antolovich M. Analytical chemistry of fruit bioflavonoids. A review. Analyst. 1997;122:11R–34R.
-
- Lu MF, Xiao ZT, Zhang HY. Where do health benefits of flavonoids come from? Insights from flavonoid targets and their evolutionary history. Biochem Biophys Res Commun. 2013;434:701–704. - PubMed
-
- Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003;133:3248S–3254S. - PubMed
-
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–9977. - PubMed
-
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

