Real-time imaging of electrical signals with an infrared FDA-approved dye
- PMID: 25229155
- PMCID: PMC4167294
- DOI: 10.1016/j.bpj.2014.07.054
Real-time imaging of electrical signals with an infrared FDA-approved dye
Abstract
Clinical methods used to assess the electrical activity of excitable cells are often limited by their poor spatial resolution or their invasiveness. One promising solution to this problem is to optically measure membrane potential using a voltage-sensitive dye, but thus far, none of these dyes have been available for human use. Here we report that indocyanine green (ICG), an infrared fluorescent dye with FDA approval as an intravenously administered contrast agent, is voltage-sensitive. The fluorescence of ICG can follow action potentials in artificial neurons and cultured rat neurons and cardiomyocytes. ICG also visualized electrical activity induced in living explants of rat brain. In humans, ICG labels excitable cells and is routinely visualized transdermally with high spatial resolution. As an infrared voltage-sensitive dye with a low toxicity profile that can be readily imaged in deep tissues, ICG may have significant utility for clinical and basic research applications previously intractable for potentiometric dyes.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
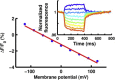
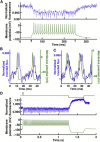
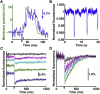
References
-
- Cohen L.B., Salzberg B.M. Optical measurement of membrane potential. Rev. Physiol. Biochem. Pharmacol. 1978;83:35–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

