Biochemical characterization of the SPATE members EspPα and EspI
- PMID: 25229188
- PMCID: PMC4179157
- DOI: 10.3390/toxins6092719
Biochemical characterization of the SPATE members EspPα and EspI
Abstract
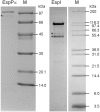
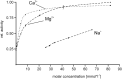
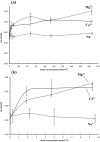
The activity of serine proteases is influenced by their substrate specificity as well as by the physicochemical conditions. Here, we present the characterization of key biochemical features of the two SPATE members EspPα and EspI from Shiga-toxin producing Escherichia coli (STEC) and enterohemorrhagic E. coli (EHEC). Both proteases show high activity at conditions mimicking the human blood stream. Optimal activities were observed at slightly alkaline pH and low millimolar concentrations of the divalent cations Ca2+ and Mg2+ at physiological temperatures indicating a function in the human host. Furthermore, we provide the first cleavage profile for EspI demonstrating pronounced specificity of this protease.
Figures





Similar articles
-
Structural and functional characterization of cleavage and inactivation of human serine protease inhibitors by the bacterial SPATE protease EspPα from enterohemorrhagic E. coli.PLoS One. 2014 Oct 27;9(10):e111363. doi: 10.1371/journal.pone.0111363. eCollection 2014. PLoS One. 2014. PMID: 25347319 Free PMC article.
-
Prevalence, biogenesis, and functionality of the serine protease autotransporter EspP.Toxins (Basel). 2012 Dec 28;5(1):25-48. doi: 10.3390/toxins5010025. Toxins (Basel). 2012. PMID: 23274272 Free PMC article. Review.
-
Enterohaemorrhagic Escherichia coli haemolysin is cleaved and inactivated by serine protease EspPα.Environ Microbiol. 2011 May;13(5):1327-41. doi: 10.1111/j.1462-2920.2011.02431.x. Epub 2011 Feb 23. Environ Microbiol. 2011. PMID: 21352460 Free PMC article.
-
Subtypes of the plasmid-encoded serine protease EspP in Shiga toxin-producing Escherichia coli: distribution, secretion, and proteolytic activity.Appl Environ Microbiol. 2007 Oct;73(20):6351-9. doi: 10.1128/AEM.00920-07. Epub 2007 Aug 17. Appl Environ Microbiol. 2007. PMID: 17704265 Free PMC article.
-
Detection of Shiga Toxin-Producing Escherichia coli from Nonhuman Sources and Strain Typing.Microbiol Spectr. 2014 Jun;2(3). doi: 10.1128/microbiolspec.EHEC-0001-2013. Microbiol Spectr. 2014. PMID: 26103970 Review.
Cited by
-
Genome sequencing and comparative genomics of enterohemorrhagic Escherichia coli O145:H25 and O145:H28 reveal distinct evolutionary paths and marked variations in traits associated with virulence & colonization.BMC Microbiol. 2017 Aug 22;17(1):183. doi: 10.1186/s12866-017-1094-3. BMC Microbiol. 2017. PMID: 28830351 Free PMC article.
-
Comparative genomic analysis of a Shiga toxin-producing Escherichia coli (STEC) O145:H25 associated with a severe pediatric case of hemolytic uremic syndrome in Davidson County, Tennessee, US.BMC Genomics. 2020 Aug 17;21(1):564. doi: 10.1186/s12864-020-06967-3. BMC Genomics. 2020. PMID: 32807093 Free PMC article.
-
Plasmids from Shiga Toxin-Producing Escherichia coli Strains with Rare Enterohemolysin Gene (ehxA) Subtypes Reveal Pathogenicity Potential and Display a Novel Evolutionary Path.Appl Environ Microbiol. 2016 Oct 14;82(21):6367-6377. doi: 10.1128/AEM.01839-16. Print 2016 Nov 1. Appl Environ Microbiol. 2016. PMID: 27542930 Free PMC article.
References
-
- Brockmeyer J., Bielaszewska M., Fruth A., Bonn M.L., Mellmann A., Humpf H.U., Karch H. Subtypes of the plasmid-encoded serine protease EspP in Shiga toxin-producing Escherichia coli: Distribution, secretion, and proteolytic activity. Appl. Environ. Microbiol. 2007;73:6351–6359. doi: 10.1128/AEM.00920-07. - DOI - PMC - PubMed
-
- Bielaszewska M., Stoewe F., Fruth A., Zhang W., Prager R., Brockmeyer J., Mellmann A., Karch H., Friedrich A.W. Shiga toxin, cytolethal distending toxin, and hemolysin repertoires in clinical Escherichia coli O91 isolates. J. Clin. Microbiol. 2009;47:2061–2066. doi: 10.1128/JCM.00201-09. - DOI - PMC - PubMed
-
- Khan A.B., Naim A., Orth D., Grif K., Mohsin M., Prager R., Dierich M.P., Wurzner R. Serine protease espP subtype alpha, but not beta or gamma, of Shiga toxin-producing Escherichia coli is associated with highly pathogenic serogroups. Int. J. Med. Microbiol. 2009;299:247–254. doi: 10.1016/j.ijmm.2008.08.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

