Liver X receptor regulation of thyrotropin-releasing hormone transcription in mouse hypothalamus is dependent on thyroid status
- PMID: 25229406
- PMCID: PMC4167690
- DOI: 10.1371/journal.pone.0106983
Liver X receptor regulation of thyrotropin-releasing hormone transcription in mouse hypothalamus is dependent on thyroid status
Abstract
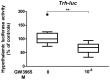
Reversing the escalating rate of obesity requires increased knowledge of the molecular mechanisms controlling energy balance. Liver X receptors (LXRs) and thyroid hormone receptors (TRs) are key physiological regulators of energetic metabolism. Analysing interactions between these receptors in the periphery has led to a better understanding of the mechanisms involved in metabolic diseases. However, no data is available on such interactions in the brain. We tested the hypothesis that hypothalamic LXR/TR interactions could co-regulate signalling pathways involved in the central regulation of metabolism. Using in vivo gene transfer we show that LXR activation by its synthetic agonist GW3965 represses the transcriptional activity of two key metabolic genes, Thyrotropin-releasing hormone (Trh) and Melanocortin receptor type 4 (Mc4r) in the hypothalamus of euthyroid mice. Interestingly, this repression did not occur in hypothyroid mice but was restored in the case of Trh by thyroid hormone (TH) treatment, highlighting the role of the triiodothyronine (T3) and TRs in this dialogue. Using shLXR to knock-down LXRs in vivo in euthyroid newborn mice, not only abrogated Trh repression but actually increased Trh transcription, revealing a potential inhibitory effect of LXR on the Hypothalamic-Pituitary-Thyroid axis. In vivo chromatin immunoprecipitation (ChIP) revealed LXR to be present on the Trh promoter region in the presence of T3 and that Retinoid X Receptor (RXR), a heterodimerization partner for both TR and LXR, was never recruited simultaneously with LXR. Interactions between the TR and LXR pathways were confirmed by qPCR experiments. T3 treatment of newborn mice induced hypothalamic expression of certain key LXR target genes implicated in metabolism and inflammation. Taken together the results indicate that the crosstalk between LXR and TR signalling in the hypothalamus centres on metabolic and inflammatory pathways.
Conflict of interest statement
Figures





References
-
- Montague CT, O’Rahilly S (2000) The perils of portliness: causes and consequences of visceral adiposity. Diabetes 49: 883–888. - PubMed
-
- Matsuzawa Y, Funahashi T, Nakamura T (1999) Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci 892: 146–154. - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846. - PubMed
-
- Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89: 2595–2600. - PubMed
-
- Jakobsson T, Treuter E, Gustafsson JA, Steffensen KR (2012) Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol Sci 33: 394–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

