Photochemistry of flavoprotein light sensors
- PMID: 25229449
- PMCID: PMC4258882
- DOI: 10.1038/nchembio.1633
Photochemistry of flavoprotein light sensors
Abstract
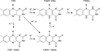
Three major classes of flavin photosensors, light oxygen voltage (LOV) domains, blue light sensor using FAD (BLUF) proteins and cryptochromes (CRYs), regulate diverse biological activities in response to blue light. Recent studies of structure, spectroscopy and chemical mechanism have provided unprecedented insight into how each family operates at the molecular level. In general, the photoexcitation of the flavin cofactor leads to changes in redox and protonation states that ultimately remodel protein conformation and molecular interactions. For LOV domains, issues remain regarding early photochemical events, but common themes in conformational propagation have emerged across a diverse family of proteins. For BLUF proteins, photoinduced electron transfer reactions critical to light conversion are defined, but the subsequent rearrangement of hydrogen bonding networks key for signaling remains highly controversial. For CRYs, the relevant photocycles are actively debated, but mechanistic and functional studies are converging. Despite these challenges, our current understanding has enabled the engineering of flavoprotein photosensors for control of signaling processes within cells.
Figures
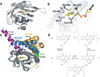



References
-
- Masuda S. Light detection and signal transduction in the BLUF photoreceptors. Plant and Cell Physiology. 2013;54:171–179. - PubMed
-
- Kennis JTM, Groot ML. Ultrafast spectroscopy of biological photoreceptors. Current Opinion in Structural Biology. 2007;17:623–630. - PubMed
-
- Losi A, Gartner W. Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochemistry and photobiology. 2011;87:491–510. - PubMed
-
- Moglich A, Yang XJ, Ayers RA, Moffat K. Structure and function of plant photoreceptors. Annual Review of Plant Biology, Vol 61. 2010;61:21–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

