β-Glucan induces reactive oxygen species production in human neutrophils to improve the killing of Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis
- PMID: 25229476
- PMCID: PMC4168232
- DOI: 10.1371/journal.pone.0107805
β-Glucan induces reactive oxygen species production in human neutrophils to improve the killing of Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis
Abstract
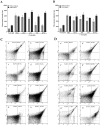
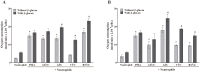
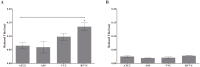
Vulvovaginal candidiasis (VVC) is among the most prevalent vaginal diseases. Candida albicans is still the most prevalent species associated with this pathology, however, the prevalence of other Candida species, such as C. glabrata, is increasing. The pathogenesis of these infections has been intensely studied, nevertheless, no consensus has been reached on the pathogenicity of VVC. In addition, inappropriate treatment or the presence of resistant strains can lead to RVVC (vulvovaginal candidiasis recurrent). Immunomodulation therapy studies have become increasingly promising, including with the β-glucans. Thus, in the present study, we evaluated microbicidal activity, phagocytosis, intracellular oxidant species production, oxygen consumption, myeloperoxidase (MPO) activity, and the release of tumor necrosis factor α (TNF-α), interleukin-8 (IL-8), IL-1β, and IL-1Ra in neutrophils previously treated or not with β-glucan. In all of the assays, human neutrophils were challenged with C. albicans and C. glabrata isolated from vulvovaginal candidiasis. β-glucan significantly increased oxidant species production, suggesting that β-glucan may be an efficient immunomodulator that triggers an increase in the microbicidal response of neutrophils for both of the species isolated from vulvovaginal candidiasis. The effects of β-glucan appeared to be mainly related to the activation of reactive oxygen species and modulation of cytokine release.
Conflict of interest statement
Figures


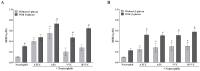


References
-
- Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD (2000) Candida vaginitis: self-reported incidence and associated costs. Sex Transm Dis 27: 230–235. - PubMed
-
- Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J (2013) Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J Low Genit Tract Dis 17: 340–345. - PubMed
-
- Colombo AL, Guimaraes T, Camargo LF, Richtmann R, Queiroz-Telles F, et al. (2013) Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis 17: 283–312. - PMC - PubMed
-
- Kennedy MA, Sobel JD (2010) Vulvovaginal Candidiasis Caused by Non-albicans Candida Species: New Insights. Curr Infect Dis Rep 12: 465–470. - PubMed
-
- Grigoriou O, Baka S, Makrakis E, Hassiakos D, Kapparos G, et al. (2006) Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. European Journal of Obstetrics & Gynecology and Reproductive Biology 126: 121–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

