Oral or transdermal opioids for osteoarthritis of the knee or hip
- PMID: 25229835
- PMCID: PMC10993204
- DOI: 10.1002/14651858.CD003115.pub4
Oral or transdermal opioids for osteoarthritis of the knee or hip
Abstract
Background: Osteoarthritis is the most common form of joint disease and the leading cause of pain and physical disability in older people. Opioids may be a viable treatment option if people have severe pain or if other analgesics are contraindicated. However, the evidence about their effectiveness and safety is contradictory. This is an update of a Cochrane review first published in 2009.
Objectives: To determine the effects on pain, function, safety, and addiction of oral or transdermal opioids compared with placebo or no intervention in people with knee or hip osteoarthritis.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL (up to 28 July 2008, with an update performed on 15 August 2012), checked conference proceedings, reference lists, and contacted authors.
Selection criteria: We included randomised or quasi-randomised controlled trials that compared oral or transdermal opioids with placebo or no treatment in people with knee or hip osteoarthritis. We excluded studies of tramadol. We applied no language restrictions.
Data collection and analysis: We extracted data in duplicate. We calculated standardised mean differences (SMDs) and 95% confidence intervals (CI) for pain and function, and risk ratios for safety outcomes. We combined trials using an inverse-variance random-effects meta-analysis.
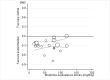
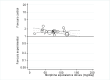
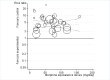
Main results: We identified 12 additional trials and included 22 trials with 8275 participants in this update. Oral oxycodone was studied in 10 trials, transdermal buprenorphine and oral tapentadol in four, oral codeine in three, oral morphine and oral oxymorphone in two, and transdermal fentanyl and oral hydromorphone in one trial each. All trials were described as double-blind, but the risk of bias for other domains was unclear in several trials due to incomplete reporting. Opioids were more beneficial in pain reduction than control interventions (SMD -0.28, 95% CI -0.35 to -0.20), which corresponds to a difference in pain scores of 0.7 cm on a 10-cm visual analogue scale (VAS) between opioids and placebo. This corresponds to a difference in improvement of 12% (95% CI 9% to 15%) between opioids (41% mean improvement from baseline) and placebo (29% mean improvement from baseline), which translates into a number needed to treat (NNTB) to cause one additional treatment response on pain of 10 (95% CI 8 to 14). Improvement of function was larger in opioid-treated participants compared with control groups (SMD -0.26, 95% CI -0.35 to -0.17), which corresponds to a difference in function scores of 0.6 units between opioids and placebo on a standardised Western Ontario and McMaster Universities Arthritis Index (WOMAC) disability scale ranging from 0 to 10. This corresponds to a difference in improvement of 11% (95% CI 7% to 14%) between opioids (32% mean improvement from baseline) and placebo (21% mean improvement from baseline), which translates into an NNTB to cause one additional treatment response on function of 11 (95% CI 7 to 14). We did not find substantial differences in effects according to type of opioid, analgesic potency, route of administration, daily dose, methodological quality of trials, and type of funding. Trials with treatment durations of four weeks or less showed larger pain relief than trials with longer treatment duration (P value for interaction = 0.001) and there was evidence for funnel plot asymmetry (P value = 0.054 for pain and P value = 0.011 for function). Adverse events were more frequent in participants receiving opioids compared with control. The pooled risk ratio was 1.49 (95% CI 1.35 to 1.63) for any adverse event (9 trials; 22% of participants in opioid and 15% of participants in control treatment experienced side effects), 3.76 (95% CI 2.93 to 4.82) for drop-outs due to adverse events (19 trials; 6.4% of participants in opioid and 1.7% of participants in control treatment dropped out due to adverse events), and 3.35 (95% CI 0.83 to 13.56) for serious adverse events (2 trials; 1.3% of participants in opioid and 0.4% of participants in control treatment experienced serious adverse events). Withdrawal symptoms occurred more often in opioid compared with control treatment (odds ratio (OR) 2.76, 95% CI 2.02 to 3.77; 3 trials; 2.4% of participants in opioid and 0.9% of participants control treatment experienced withdrawal symptoms).
Authors' conclusions: The small mean benefit of non-tramadol opioids are contrasted by significant increases in the risk of adverse events. For the pain outcome in particular, observed effects were of questionable clinical relevance since the 95% CI did not include the minimal clinically important difference of 0.37 SMDs, which corresponds to 0.9 cm on a 10-cm VAS.
Conflict of interest statement
None.
Figures
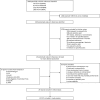
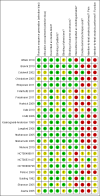
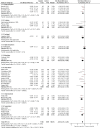



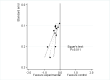










Update of
-
Oral or transdermal opioids for osteoarthritis of the knee or hip.Cochrane Database Syst Rev. 2009 Oct 7;(4):CD003115. doi: 10.1002/14651858.CD003115.pub3. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2014 Sep 17;(9):CD003115. doi: 10.1002/14651858.CD003115.pub4. PMID: 19821302 Updated.
References
References to studies included in this review
Afilalo 2010 {published data only}
-
- Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Hove I, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double‐blind, placebo‐ and active‐controlled phase III study. Clinical Drug Investigation 2010;30(8):489‐505. - PubMed
-
- Grünenthal GmbH. A study to evaluate the efficacy and safety of cg5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. clinicaltrials.gov/show/NCT00486811 (accessed 16 August 2014).
-
- Kelly K, Etropolski M, Kuperwasser B, Okamoto A, Steup A, vanHove I, et al. Similar analgesic effect and improved tolerability of tapentadol extended release versus oxycodone controlled release for the management of chronic osteoarthritis knee pain: results from a randomized, double‐blind, phase3 trial. Proceedings of the Annual EULAR Conference; 2009 Jun 10‐13; Copenhagen, Denmark.
-
- Kelly K, Lange B, Etropolski M, Kuperwasser B, Okamoto A, vanHove I, et al. Dose stability of tapentadol extended release (ER) for the relief of moderate‐to‐severe chronic osteoarthritic knee pain. Proceedings of the 26th Annual Meeting of the American Academy of Pain Medicine; 2010 Feb 3‐6; San Antonio, TX.
-
- Lange B, Kuperwasser B, Okamoto A, Häufel T, Ashworth J. Efficacy and safety of tapentadol prolonged release (PR) based on prior experience in patients with chronic osteoarthritis knee pain. Annals of Rheumatic Diseases 2010;69:287.
Breivik 2010 {published data only}
-
- Breivik H, Ljosaa TM, Stengaard‐Pedersen K, Persson J, Aro H, Villumsen J, et al. A 6‐months, randomised, placebo‐controlled evaluation of efficacy and tolerability of a low‐dose 7‐day buprenorphine transdermal patch in osteoarthritis patients naive to potent opioids. Scandinavian Journal of Pain 2010;1:122‐41. - PubMed
Caldwell 2002 {published data only}
-
- Caldwell JR, Rapoport RJ, Davis JC, Offenberg HL, Marker HW, Roth SH, et al. Efficacy and safety of a once‐daily morphine formulation in chronic, moderate‐to‐severe osteoarthritis pain: results from a randomized, placebo‐controlled, double‐blind trial and an open‐label extension trial. Journal of Pain and Symptom Management 2002;23(4):278‐91. - PubMed
Chindalore 2005 {published data only}
-
- Chindalore VL, Craven RA, Yu KP, Butera PG, Burns LH, Friedmann N. Adding ultra low‐dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of oxytrex. Journal of Pain 2005;6(6):392‐9. - PubMed
Etropolski 2011 {published data only}
-
- Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Advances in Therapy 2011;28(5):401‐17. - PubMed
-
- Johnson, Johnson. A study to compare the frequency of constipation symptoms with tapentadol immediate release (IR) treatment versus oxycodone IR treatment in patients with end‐stage joint disease. clinicaltrials.gov/show/NCT00784277 (accessed 16 August 2014).
Fidelholtz 2011 {published data only}
-
- Fidelholtz J, Tark M, Spierings E, Wolfram G, Annis K, Smith MD, et al. A phase 3 placebo‐ and oxycodone‐controlled study of tanezumab in adults with osteoarthritis. Proceedings of the ACR/ARHP Scientific Meeting; 2011 Nov 9‐11; Chicago.
-
- Pfizer. Tanezumab in osteoarthritis of the hip or knee. clinicaltrials.gov/show/NCT00985621 (accessed 16 August 2014).
Friedmann 2011 {published data only}
-
- Friedmann N, Klutzaritz V, Webster L. Efficacy and safety of an extended‐release oxycodone (Remoxy®) formulation in patients with moderate to severe osteoarthritic pain. Journal of Opioid Management 2011;7(3):193‐202. - PubMed
Hartrick 2009 {published data only}
-
- Hartrick C, Hove I, Stegmann JU, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end‐stage joint disease: a 10‐day, phase III, randomized, double‐blind, active‐ and placebo‐controlled study. Clinical Therapeutics 2009;31(2):260‐71. - PubMed
-
- Johnson, Johnson. A study to evaluate the effectiveness and safety of multiple doses of tapentadol (CG5503) in patients awaiting joint replacement surgery. clinicaltrials.gov/show/NCT00361582 (accessed 16 August 2014).
Katz 2010 {published data only}
-
- Alpharma. A study of embeda (Kadian NT, ALO‐01) in subjects with pain due to osteoarthritis of the hip or knee. clinicaltrials.gov/show/NCT00420992 (accessed 16 August 2014).
-
- Jones JB, Wagner G, Morris D, Stauffer J. Efficacy of morphine sulfate extended‐release with sequestered naltrexone hydrochloride (ALO‐01) in patients with chronic, moderate to severe pain of osteoarthritis of the hip or knee. Proceedings of the American College of Rheumatology Annual Scientific Meeting; 2008 Oct 24‐29; San Francisco.
-
- Katz N, Hale M, Morris D, Stauffer J. Morphine sulfate and naltrexone hydrochloride extended release capsules in patients with chronic osteoarthritis pain. Postgraduate Medicine 2010;122(4):112‐28. - PubMed
-
- Katz N, Setnik B, Webster L. Safety profile of EMBEDA™ (morphine sulfate and naltrexone hydrochloride) Extended Release Capsules in older patients. Proceedings of the 29th Annual Scientific Meeting of the American Pain Society; 2010 May 6‐8; Baltimore, MD.
Kivitz 2006 {published data only}
-
- Kivitz A, Ma C, Ahdieh H, Galer BS. A 2‐week, multicenter, randomized, double‐blind, placebo‐controlled, dose‐ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee. Clinical Therapeutics 2006;28(3):352‐64. - PubMed
Kjaersgaard‐Andersen 1990 {published data only}
-
- Kjaersgaard‐Andersen P, Nafei A, Skov O, Madsen F, Andersen HM, Kroner K, et al. Codeine plus paracetamol versus paracetamol in longer‐term treatment of chronic pain due to osteoarthritis of the hip. A randomised, double‐blind, multi‐centre study. Pain 1990;43(3):309‐18. - PubMed
Langford 2006 {published data only}
-
- Langford R, McKenna F, Ratcliffe S, Vojtassak J, Richarz U. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis: a randomized, placebo‐controlled trial. Arthritis and Rheumatism 2006;54(6):1829‐37. - PubMed
Markenson 2005 {published data only}
-
- Markenson JA, Croft J, Zhang PG, Richards P. Treatment of persistent pain associated with osteoarthritis with controlled‐release oxycodone tablets in a randomized controlled clinical trial. Clinical Journal of Pain 2005;21(6):524‐35. - PubMed
Matsumoto 2005 {published data only}
-
- Matsumoto AK, Babul N, Ahdieh H. Oxymorphone extended‐release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double‐blind, placebo‐ and active‐controlled phase III trial. Pain Medicine 2005;6(5):357‐66. - PubMed
Munera 2010 {published data only}
-
- Munera C, Drehobl M, Sessler NE, Landau C. A randomized, placebo‐controlled, double‐blinded, parallel‐group, 5‐week study of buprenorphine transdermal system in adults with osteoarthritis. Journal of Opioid Management 2010;6(3):193‐202. - PubMed
-
- Purdue. Safety and efficacy of the buprenorphine transdermal delivery system in subjects with osteoarthritis pain. clinicaltrials.gov/show/NCT00314652 (accessed 16 August 2014).
NCT00486811 {published data only}
-
- Grünenthal. A study to evaluate the efficacy and safety of CG5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. clinicaltrials.gov/show/NCT00486811 (accessed 16 August 2014).
NCT00531427 {published data only}
-
- Purdue. A study to evaluate the efficacy and safety of CG5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. clinicaltrials.gov/show/NCT00531427 (accessed 16 August 2014).
NCT00980798 {published data only}
-
- Janssen‐Cilag. Placebo‐controlled trial with OROS hydromorphone hydrochloride to treat patients with moderate to severe pain induced by osteoarthritis of the hip or the knee. clinicaltrials.gov/show/NCT00980798 (accessed 16 August 2014).
Peloso 2000 {published data only}
-
- Peloso PM, Bellamy N, Bensen W, Thomson GTD, Harsanyi Z, Babul N, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. Journal of Rheumatology 2000;27(3):764‐71. - PubMed
Quiding 1992 {published data only}
-
- Quiding H, Grimstad J, Rusten K, Stubhaug A, Bremnes J, Breivik H. Ibuprofen plus codeine, ibuprofen, and placebo in a single‐ and multidose cross‐over comparison for coxarthrosis pain. Pain 1992;50(3):303‐7. - PubMed
-
- Öhrvik, J. Nonparametric methods in crossover trials. Biometrical Journal 1998;7:771‐89.
Shannon 2005 {published data only}
-
- Landau CJ, Shannon M, Kivitz AJ, Sessler NE, Xia Y, Ripa SR. Buprenorphine transdermal system in chronic pain due to osteoarthritis. Arthritis and Rheumatism 2008;58(Suppl 9):866.
-
- Purdue. Safety and efficacy of buprenorphine transdermal system in subjects with moderate to severe osteoarthritis of hip or knee. clinicaltrials.gov/show/NCT00313846 (accessed 16 August 2014).
-
- Shannon MJ, Kivitz AJ, Landau CJ, Sessler NE, Xia Y, Ripa SR. Buprenorphine transdermal system in chronic pain due to osteoarthritis. Archives of Physical Medicine and Rehabilitation 2005;86:E32.
Zautra 2005 {published data only}
-
- Zautra AJ, Smith BW. Impact of controlled‐release oxycodone on efficacy beliefs and coping efforts among osteoarthritis patients with moderate to severe pain. Clinical Journal of Pain 2005;21(6):471‐7. - PubMed
References to studies excluded from this review
Adams 2006 {published data only}
-
- Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. Journal of Pain and Symptom Management 2006;31(5):465‐76. - PubMed
Andrei 1984 {published data only}
-
- Andrei A, Schiaroli G, Algeri R, Valentini P. Recent data on antiinflammatory and analgesic treatment of degenerative arthropathies. Double‐blind controlled clinical trial. [Italian]. Archivio di Medicina Interna 1984;36(4):245‐56.
Boureau 1990 {published data only}
-
- Boureau F, Delecoeuillerie G, Orvain J. Comparative study of the efficacy and tolerance of 2 dosages of the paracetamol 400 mg codeine 25 mg association versus paracetamol 1000 mg in non‐inflammatory rheumatic pain. Rhumatologie Revue International de Rhumatologie 1990;20(1):41‐7.
Boyer 2012 {published data only}
-
- Boyer KA, Angst MS, Asay J, Giori NJ, Andriacchi TP. Sensitivity of gait parameters to the effects of anti‐inflammatory and opioid treatments in knee osteoarthritis patients. Journal of Orthopaedic Research 2012;30(7):1118‐24. - PubMed
Brooks 1982 {published data only}
-
- Brooks PM, Dougan MA, Mugford S, Meffin E. Comparative effectiveness of 5 analgesics in patients with rheumatoid arthritis and osteoarthritis. Journal of Rheumatology 1982;9(5):723‐6. - PubMed
Burch 2004 {published data only}
-
- Burch F, Codding C, Patel N, Sheldon E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open‐label effectiveness trial. Osteoarthritis and Cartilage 2004;12(3):253‐5. - PubMed
Caldwell 1999 {published data only}
-
- Caldwell JR, Hale ME, Boyd RE, Hague JM, Iwan T, Shi M, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial. Journal of Rheumatology 1999;26(4):862‐9. - PubMed
Choquette 2008 {published data only}
-
- Choquette D, McCarthy TG, Rodrigues JFN, Kelly AJ, Camacho F, Horbay GLA, et al. Transdermal fentanyl improves pain control and functionality in patients with osteoarthritis: an open‐label Canadian trial. Clinical Rheumatology 2008;27(5):587‐95. - PubMed
Conaghan 2011 {published data only}
-
- Conaghan PG, O'Brien CM, Wilson M, Schofield, JP. Transdermal buprenorphine plus oral paracetamol vs an oral codeine‐paracetamol combination for osteoarthritis of hip and/or knee: a randomised trial. Osteoarthritis & Cartilage 2011;19(8):930‐8. - PubMed
Corsinovi 2009 {published data only}
-
- Corsinovi L, Martinelli E, Fonte G, Astengo M, Sona, A, Gatti A, et al. Efficacy of oxycodone/acetaminophen and codeine/acetaminophen vs. conventional therapy in elderly women with persistent, moderate to severe osteoarthritis‐related pain. Archives of Gerontology & Geriatrics 2009;49(3):378‐82. - PubMed
Doak 1992 {published data only}
-
- Doak W, Hosie J, Hossain M, James IGV, Reid I, Miller AJ. A novel combination of ibuprofen and codeine phosphate in the treatment of osteoarthritis: a double‐blind placebo controlled study. Journal of Drug Development 1992;4(4):179‐87.
Fancourt 1984 {published data only}
-
- Fancourt GJ, Flavell Matts SG. A double‐blind comparison of meptazinol versus placebo in chronic rheumatoid arthritis and osteoarthritis. Current Medical Research and Opinion 1984;9(3):184‐91. - PubMed
Friedmann 2011b {published data only}
-
- Friedmann N, Klutzaritz V, Webster L. Long‐term safety of Remoxy® (extended‐release oxycodone) in patients with moderate to severe chronic osteoarthritis or low back pain. Pain Medicine 2011;12(5):755‐60. - PubMed
Gazi 2005 {published data only}
-
- Gazi MCB, Machado Issy A, Kimiko Sakata R. Intra‐articular bupivacaine and morphine for knee osteoarthritis analgesia. Comparative study. Revista Brasileira de Anestesiologia 2005;55(5):491‐9. - PubMed
Hale 2007 {published data only}
-
- Hale M, Tudor IC, Khanna S, Thipphawong J. Efficacy and tolerability of once‐daily OROS hydromorphone and twice‐daily extended‐release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: results of a 6‐week, randomized, open‐label, noninferiority analysis. Clinical Therapeutics 2007;29(5):874‐88. - PubMed
James 2010 {published data only}
-
- James IGV, O'Brien CM, McDonald CJ. A randomized, double‐blind, double‐dummy comparison of the efficacy and tolerability of low‐dose transdermal buprenorphine (BuTrans seven‐day patches) with buprenorphine sublingual tablets (Temgesic) in patients with osteoarthritis pain. Journal of Pain & Symptom Management 2010;40(2):266‐78. - PubMed
Katz 2010b {published data only}
-
- Katz N, Sun S, Johnson F, Stauffer J. ALO‐01 (morphine sulfate and naltrexone hydrochloride) extended‐release capsules in the treatment of chronic pain of osteoarthritis of the hip or knee: pharmacokinetics, efficacy, and safety. Journal of Pain 2010;11(4):303‐11. - PubMed
Le Loet 2005 {published data only}
McIlwain 2005 {published data only}
-
- McIlwain H, Ahdieh H. Safety, tolerability, and effectiveness of oxymorphone extended release for moderate to severe osteoarthritis pain: a one‐year study. American Journal of Therapeutics 2005;12(2):106‐12. - PubMed
Mitchell 1984 {published data only}
-
- Mitchell H, Cunningham TJ, Mathews JD, Muirden KD. Further look at dextropropoxyphene with or without paracetamol in the treatment of arthritis. Medical Journal of Australia 1984;140(4):224‐5. - PubMed
Neubauer 1983 {published data only}
-
- Neubauer M, Bach GL. Short‐term therapy of painful muscular disorders. Results of a multicenter double‐blind test of 2 new suppository preparations with and without codeine. Fortschritte der Medizin 1983;101(21):1009‐13. - PubMed
Rosenthal 2007 {published data only}
-
- Rosenthal M, Moore P, Groves E, Iwan T, Greenberg Schlosser L, Dziewanowska Z, et al. Sleep improves when patients with chronic OA pain are managed with morning dosing of once a day extend‐release morphine sulfate (AVINZA): findings from a pilot study. Journal of Opioid Management 2007;3(3):145‐53. - PubMed
Roth 2000 {published data only}
-
- Roth SH, Fleischmann RM, Burch FX, Dietz F, Bockow B, Rapoport RJ, et al. Around‐the‐clock, controlled‐release oxycodone therapy for osteoarthritis‐related pain: placebo‐controlled trial and long‐term evaluation. Archives of Internal Medicine 2000;160(6):853‐60. - PubMed
Salzman 1983 {published data only}
-
- Salzman RT, Brobyn RD. Long‐term comparison of suprofen and propoxyphene in patients with osteoarthritis. Pharmacology 1983;27 Suppl 1:55‐64. - PubMed
Tassain 2003 {published data only}
-
- Tassain V, Attal N, Fletcher D, Brasseur L, Degieux P, Chauvin M, et al. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non‐cancer pain. Pain 2003;104(1‐2):389‐400. - PubMed
Torres 2001 {published data only}
-
- Torres Huerta JC, Hernandez Santos JR, Tenopala Villegas S. Transdermal fentanyl in patients with nononcological chronic pain [Spanish]. Revista Mexicana de Anestesiologia 2001;24(2):65‐8.
Vignon 1999 {published data only}
-
- Vignon E, Bannwarth B, Conrozier T, Derobert E, Verdoncq B. Multicenter, double‐blind, clinical trial comparing two tablets bid to one tablet qid of the same acetaminophen‐dextropropoxyphen‐caffeine combination in patients with osteoarthritis [French]. Semaine des Hopitaux 1999;75(13‐14):419‐25.
Vlok 1987 {published data only}
-
- Vlok GJ, Vuren JP. Comparison of a standard ibuprofen treatment regimen with a new ibuprofen/paracetamol/codeine combination in chronic osteo‐arthritis. South African Medical Journal [Suid Afrikaanse Tydskrif vir Geneeskunde] 1987;Suppl:1, 4‐6. - PubMed
Vorsanger 2011 {published data only}
-
- Vorsanger G, Xiang J, Biondi D, Upmalis D, Delfgaauw J, Allard R, et al. Post hoc analyses of data from a 90‐day clinical trial evaluating the tolerability and efficacy of tapentadol immediate release and oxycodone immediate release for the relief of moderate to severe pain in elderly and nonelderly patients. Pain Research & Management 2011;16(4):245‐51. - PMC - PubMed
Wallace 1994 {published data only}
-
- Wallace WA, Elliott CA, Price VH. A combination of ibuprofen and codeine phosphate provides superior analgesia to ibuprofen alone in osteoarthritis. British Journal of Clinical Research 1994;5:33‐46.
Wang 1965 {published data only}
-
- Wang RI. Analgesic effectiveness of new propoxyphene preparations. Journal of New Drugs 1965;5(3):171‐6. - PubMed
Wild 2010 {published data only}
-
- Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, Lange B, et al. Long‐term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Practice 2010;10(5):416‐27. - PubMed
References to studies awaiting assessment
Kroner 1991 {published data only}
-
- Kroner K, Hansen TB, Harving S, Hvass I, Madsen F, Nafei A, et al. Individually dosed codeine plus paracetamol versus paracetamol in long‐term treatment of chronic pain due to arthrosis of the hip ‐ a randomised, double blind, multicenter study. Acta Orthopaedica Scandinavica, Supplement 1991;62(246):43.
Additional references
Altman 1986
-
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis and Rheumatism 1986;29:1039‐49. - PubMed
Altman 1996
-
- Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis and Cartilage 1996;4(4):217‐43. - PubMed
Avouac 2007
-
- Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta‐analysis of randomized controlled trials. Osteoarthritis and Cartilage 2007;15(8):957‐65. - PubMed
Bellamy 1995
-
- Bellamy N. Outcome measurement in osteoarthritis clinical trials. Journal of Rheumatology Supplement 1995;43:49‐51. - PubMed
British Pain Society 2010
-
- British Pain Society. Opioids for persistent pain: good practice, 2010. www.britishpainsociety.org/book_opioid_main.pdf (accessed 16 August 2014).
Caldwell 2005
Cepeda 2006
Chinn 2000
-
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐31. - PubMed
Clegg 2006
-
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. New England Journal of Medicine 2006;354(8):795‐808. - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988.
da Costa 2012a
-
- Costa BR, Rutjes AWS, Johnston BC, Reichenbach S, Nüesch E, Tonia T, et al. Methods to convert continuous outcomes into odds ratios of treatment response and numbers needed to treat: meta‐epidemiological study. International Journal of Epidemiology 2012;41(5):1445‐59. - PubMed
da Costa 2012b
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dickersin 1994
Eccles 1998
Egger 2001
-
- Egger M, Smith GD. Principles of and procedures for systematic reviews. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. London: BMJ Books, 2001:23‐42.
Egger 2003
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment (Winchester, England) 2003;7(1):1‐76. - PubMed
Eriksen 2006
-
- Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non‐cancer pain: an epidemiological study. Pain 2006;125(1‐2):172‐9. - PubMed
Furlan 2006
Gehling 2011
-
- Gehling M, Hermann B, Tryba M. Meta‐analysis of dropout rates in randomized controlled clinical trials: opioid analgesia for osteoarthritis pain. Schmerz 2011;25(3):296‐305. - PubMed
Gossop 1990
-
- Gossop M. The development of a Short Opiate Withdrawal Scale (SOWS). Addictive Behaviors 1990;15(5):487‐90. - PubMed
Guyatt 2008
Gøtzsche 2007
-
- Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta‐analyses that use standardized mean differences. JAMA 2007;298(4):430‐7. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hochberg 2012
-
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care and Research 2012;64(4):465‐74. - PubMed
Jüni 2001
Jüni 2006
-
- Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Practice & Research. Clinical Rheumatology 2006;20(4):721‐40. - PubMed
Kalso 2004
-
- Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain 2004;112(3):372‐80. - PubMed
Loeser 2001
-
- Loeser JD, Butler SH, Chapman CR, Turk DC. Bonica's Management of Pain. 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2001.
Maier 2002
-
- Maier C, Hildebrandt J, Klinger R, Henrich‐Eberl C, Lindena G, MONTAS Study Group. Morphine responsiveness, efficacy and tolerability in patients with chronic non‐tumor associated pain ‐ results of a double‐blind placebo‐controlled trial (MONTAS). Pain 2002;97(3):223‐33. - PubMed
NICE 2008
-
- NICE clinical guideline 59. Osteoarthritis ‐ the care and management of osteoarthritis in adults. www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf (accessed 16 August 2014).
Nüesch 2009
Nüesch 2010
Pham 2004
-
- Pham T, Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT‐OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis and Cartilage 2004;12(5):389‐99. - PubMed
Reichenbach 2007
-
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, et al. Meta‐analysis: chondroitin for osteoarthritis of the knee or hip. Annals of Internal Medicine 2007;146(8):580‐90. - PubMed
Reichenbach 2010
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rutjes 2009a
Rutjes 2009b
Rutjes 2010
Rutjes 2012
-
- Rutjes AW, Jüni P, Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(3):180‐91. - PubMed
Rücker 2008
Schug 2006
-
- Schug SA, Gandham N. Opioids: clinical use. In: McMahon S, Klotzenburg M editor(s). Wall and Melzack's Textbook of Pain. 5th Edition. Oxford: Elsevier Limited, 2006:443‐57.
Shang 2005
-
- Shang A, Huwiler‐Muntener K, Nartey L, Juni P, Dorig S, Sterne JA, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo‐controlled trials of homoeopathy and allopathy. Lancet 2005;366(9487):726‐32. - PubMed
Stein 1996
Sterne 2001
-
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Thompson 1999
-
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. - PubMed
Von Korff 2004
-
- Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind?. Pain 2004;109(3):207‐9. - PubMed
Wandel 2010
Zhang 2008
-
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence‐based, expert consensus guidelines. Osteoarthritis and Cartilage 2008;16(2):137‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

