The INTERVAL trial to determine whether intervals between blood donations can be safely and acceptably decreased to optimise blood supply: study protocol for a randomised controlled trial
- PMID: 25230735
- PMCID: PMC4177700
- DOI: 10.1186/1745-6215-15-363
The INTERVAL trial to determine whether intervals between blood donations can be safely and acceptably decreased to optimise blood supply: study protocol for a randomised controlled trial
Abstract
Background: Ageing populations may demand more blood transfusions, but the blood supply could be limited by difficulties in attracting and retaining a decreasing pool of younger donors. One approach to increase blood supply is to collect blood more frequently from existing donors. If more donations could be safely collected in this manner at marginal cost, then it would be of considerable benefit to blood services. National Health Service (NHS) Blood and Transplant in England currently allows men to donate up to every 12 weeks and women to donate up to every 16 weeks. In contrast, some other European countries allow donations as frequently as every 8 weeks for men and every 10 weeks for women. The primary aim of the INTERVAL trial is to determine whether donation intervals can be safely and acceptably decreased to optimise blood supply whilst maintaining the health of donors.
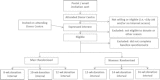
Methods/design: INTERVAL is a randomised trial of whole blood donors enrolled from all 25 static centres of NHS Blood and Transplant. Recruitment of about 50,000 male and female donors started in June 2012 and was completed in June 2014. Men have been randomly assigned to standard 12-week versus 10-week versus 8-week inter-donation intervals, while women have been assigned to standard 16-week versus 14-week versus 12-week inter-donation intervals. Sex-specific comparisons will be made by intention-to-treat analysis of outcomes assessed after two years of intervention. The primary outcome is the number of blood donations made. A key secondary outcome is donor quality of life, assessed using the Short Form Health Survey. Additional secondary endpoints include the number of 'deferrals' due to low haemoglobin (and other factors), iron status, cognitive function, physical activity, and donor attitudes. A comprehensive health economic analysis will be undertaken.
Discussion: The INTERVAL trial should yield novel information about the effect of inter-donation intervals on blood supply, acceptability, and donors' physical and mental well-being. The study will generate scientific evidence to help formulate blood collection policies in England and elsewhere.
Trial registration: Current Controlled Trials ISRCTN24760606, 25 January 2012.
Figures
Comment in
-
Lessons from the INTERVAL study.Lancet. 2018 Jun 30;391(10140):2604-2605. doi: 10.1016/S0140-6736(18)30795-5. Lancet. 2018. PMID: 30070219 No abstract available.
References
-
- National Blood Service . Guidelines for the Blood Transfusion Services in the United Kingdom. 8. London: The Stationary Office; 2013.
-
- European Directorate for the Quality of Medicine & Health Care, Council of Europe . Guide to the Preparation, Use and Quality Assurance of Blood Components. Recommendation No. R (95) 15. 17. France: Strasbourg; 2013.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical