Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models
- PMID: 25230742
- PMCID: PMC4240496
- DOI: 10.3892/or.2014.3487
Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models
Abstract
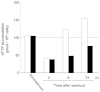
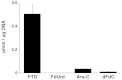
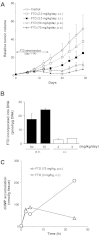
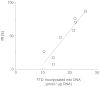
TAS-102 is a novel oral nucleoside antitumor agent containing trifluridine (FTD) and tipiracil hydrochloride (TPI). The compound improves overall survival of colorectal cancer (CRC) patients who are insensitive to standard chemotherapies. FTD possesses direct antitumor activity since it inhibits thymidylate synthase (TS) and is itself incorporated into DNA. However, the precise mechanisms underlying the incorporation into DNA and the inhibition of TS remain unclear. We found that FTD-dependent inhibition of TS was similar to that elicited by fluorodeoxyuridine (FdUrd), another clinically used nucleoside analog. However, washout experiments revealed that FTD-dependent inhibition of TS declined rapidly, whereas FdUrd activity persisted. The incorporation of FTD into DNA was significantly higher than that of other antitumor nucleosides. Additionally, orally administered FTD had increased antitumor activity and was incorporated into DNA more effectively than continuously infused FTD. When TAS-102 was administered, FTD gradually accumulated in tumor cell DNA, in a TPI-independent manner, and significantly delayed tumor growth and prolonged survival, compared to treatment with 5-FU derivatives. TAS-102 reduced the Ki-67-positive cell fraction, and swollen nuclei were observed in treated tumor tissue. The amount of FTD incorporation in DNA and the antitumor activity of TAS-102 in xenograft models were positively and significantly correlated. These results suggest that TAS-102 exerts its antitumor activity predominantly due to its DNA incorporation, rather than as a result of TS inhibition. The persistence of FTD in the DNA of tumor cells treated with TAS-102 may underlie its ability to prolong survival in cancer patients.
Figures



References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. - PubMed
-
- Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. - PubMed
-
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

