Biomechanical factors in atherosclerosis: mechanisms and clinical implications
- PMID: 25230814
- PMCID: PMC4810806
- DOI: 10.1093/eurheartj/ehu353
Biomechanical factors in atherosclerosis: mechanisms and clinical implications
Abstract
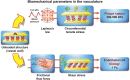
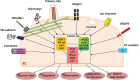
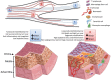
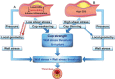
Blood vessels are exposed to multiple mechanical forces that are exerted on the vessel wall (radial, circumferential and longitudinal forces) or on the endothelial surface (shear stress). The stresses and strains experienced by arteries influence the initiation of atherosclerotic lesions, which develop at regions of arteries that are exposed to complex blood flow. In addition, plaque progression and eventually plaque rupture is influenced by a complex interaction between biological and mechanical factors-mechanical forces regulate the cellular and molecular composition of plaques and, conversely, the composition of plaques determines their ability to withstand mechanical load. A deeper understanding of these interactions is essential for designing new therapeutic strategies to prevent lesion development and promote plaque stabilization. Moreover, integrating clinical imaging techniques with finite element modelling techniques allows for detailed examination of local morphological and biomechanical characteristics of atherosclerotic lesions that may be of help in prediction of future events. In this ESC Position Paper on biomechanical factors in atherosclerosis, we summarize the current 'state of the art' on the interface between mechanical forces and atherosclerotic plaque biology and identify potential clinical applications and key questions for future research.
Keywords: Atherosclerosis; Blood flow; Endothelial cell; Haemodynamics; Mechanotransduction; Plaque rupture.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2014. For permissions please email: journals.permissions@oup.com.
Figures
References
-
- Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr Vasc Pharmacol. 2003;1:41–58. - PubMed
-
- Steinman DA. Simulated pathline visualization of computed periodic blood flow patterns. J Biomech. 2000;33:623–628. - PubMed
-
- Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99:276–283. - PubMed
-
- Chouinard-Pelletier G, Jahnsen ED, Jones EA. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis. 2013;16:71–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

