Critical role of insulin‑like growth factor binding protein‑5 in methamphetamine‑induced apoptosis in cardiomyocytes
- PMID: 25230843
- PMCID: PMC4214346
- DOI: 10.3892/mmr.2014.2572
Critical role of insulin‑like growth factor binding protein‑5 in methamphetamine‑induced apoptosis in cardiomyocytes
Abstract
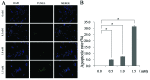
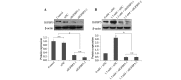
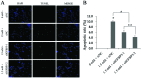
Methamphetamine (MA) is a highly abused amphetamine‑like psychostimulant. At present, the mechanisms underlying MA‑induced cardiotoxicity are poorly understood. The cardiotoxic effects have yet not been clearly elucidated with respect to the apoptotic pathway. Insulin‑like growth factor binding protein‑5 (IGFBP5) is important for cell growth control and the induction of apoptosis. The aim of the present study was to analyze whether IGFBP5 is involved in MA‑induced apoptosis as a novel target. MA‑induced apoptosis was observed in neonatal rat ventricular myocytes (NRVMs) in a concentration‑dependent manner using a terminal deoxyribonucleotide transferase‑mediated dUTP nick end‑labeling assay. Using reverse transcription polymerase chain reaction and western blotting, MA was demonstrated to induce concentration‑dependent increases in the expression of IGFBP5. Silencing IGFBP5 with small interfering RNA significantly reduced apoptosis and suppressed the expression of caspase‑3 in NRVMs following treatment with MA. To the best of our knowledge, the present study provided the first evidence suggesting that IGFBP5 is a potential therapeutic target in MA‑induced apoptosis in vitro, providing a foundation for future in vivo studies.
Figures


Similar articles
-
Insulin-like growth factor binding protein 5 (IGFBP5) mediates methamphetamine-induced dopaminergic neuron apoptosis.Toxicol Lett. 2014 Nov 4;230(3):444-53. doi: 10.1016/j.toxlet.2014.08.010. Epub 2014 Aug 13. Toxicol Lett. 2014. PMID: 25127757
-
Remodeling of ion channel expression may contribute to electrophysiological consequences caused by methamphetamine in vitro and in vivo.Biochem Biophys Res Commun. 2014 Jan 10;443(2):441-6. doi: 10.1016/j.bbrc.2013.11.114. Epub 2013 Dec 8. Biochem Biophys Res Commun. 2014. PMID: 24326070
-
DNA damage-inducible transcript 4 (DDIT4) mediates methamphetamine-induced autophagy and apoptosis through mTOR signaling pathway in cardiomyocytes.Toxicol Appl Pharmacol. 2016 Mar 15;295:1-11. doi: 10.1016/j.taap.2016.01.017. Epub 2016 Jan 26. Toxicol Appl Pharmacol. 2016. PMID: 26825372
-
Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1α/Drp1 pathway.Toxicol Appl Pharmacol. 2019 Apr 15;369:73-81. doi: 10.1016/j.taap.2019.02.016. Epub 2019 Mar 1. Toxicol Appl Pharmacol. 2019. PMID: 30831132
-
ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity.J Mol Med (Berl). 2009 Apr;87(4):401-10. doi: 10.1007/s00109-008-0434-z. Epub 2009 Jan 13. J Mol Med (Berl). 2009. PMID: 19139834
Cited by
-
Repeated exposure to methamphetamine induces sex-dependent hypersensitivity to ischemic injury in the adult rat heart.PLoS One. 2017 Jun 2;12(6):e0179129. doi: 10.1371/journal.pone.0179129. eCollection 2017. PLoS One. 2017. PMID: 28575091 Free PMC article.
-
Short term methylphenidate treatment does not increase myocardial injury in the ischemic rat heart.Physiol Res. 2020 Nov 16;69(5):803-812. doi: 10.33549/physiolres.934368. Epub 2020 May 29. Physiol Res. 2020. PMID: 32469230 Free PMC article.
-
Increased B-type-natriuretic peptide promotes myocardial cell apoptosis via the B-type-natriuretic peptide/long non-coding RNA LSINCT5/caspase-1/interleukin 1β signaling pathway.Mol Med Rep. 2015 Nov;12(5):6761-7. doi: 10.3892/mmr.2015.4247. Epub 2015 Aug 25. Mol Med Rep. 2015. PMID: 26323562 Free PMC article.
-
Insulin-Like Growth Factor Binding Protein-5 in Physiology and Disease.Front Endocrinol (Lausanne). 2020 Mar 3;11:100. doi: 10.3389/fendo.2020.00100. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32194505 Free PMC article. Review.
-
IGFBP5 antisense and short hairpin RNA (shRNA) constructs improve erectile function by inducing cavernosum angiogenesis in diabetic mice.Andrology. 2023 Feb;11(2):358-371. doi: 10.1111/andr.13234. Epub 2022 Aug 7. Andrology. 2023. PMID: 35866351 Free PMC article.
References
-
- Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. - PubMed
-
- Shrem MT, Halkitis PN. Methamphetamine abuse in the United States: contextual, psychological and sociological considerations. J Health Psychol. 2008;13:669–679. - PubMed
-
- Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines: an update. Arch Toxicol. 2012;86:1167–1231. - PubMed
-
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–69. - PubMed
-
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

