Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma
- PMID: 25230881
- PMCID: PMC4747023
- DOI: 10.1038/nrc3811
Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma
Abstract
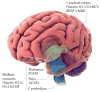
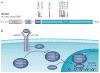
Diffuse high-grade gliomas (HGGs) of childhood are a devastating spectrum of disease with no effective cures. The two-year survival for paediatric HGG ranges from 30%, for tumours arising in the cerebral cortex, to less than 10% for diffuse intrinsic pontine gliomas (DIPGs), which arise in the brainstem. Recent genome-wide studies provided abundant evidence that unique selective pressures drive HGG in children compared to adults, identifying novel oncogenic mutations connecting tumorigenesis and chromatin regulation, as well as developmental signalling pathways. These new genetic findings give insights into disease pathogenesis and the challenges and opportunities for improving patient survival in these mostly incurable childhood brain tumours.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

