Cytoprotective-selective activated protein C therapy for ischaemic stroke
- PMID: 25230930
- PMCID: PMC4356129
- DOI: 10.1160/TH14-05-0448
Cytoprotective-selective activated protein C therapy for ischaemic stroke
Abstract
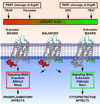
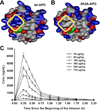
Despite years of research and efforts to translate stroke research to clinical therapy, ischaemic stroke remains a major cause of death, disability, and diminished quality of life. Primary and secondary preventive measures combined with improved quality of care have made significant progress. However, no novel drug for ischaemic stroke therapy has been approved in the past decade. Numerous studies have shown beneficial effects of activated protein C (APC) in rodent stroke models. In addition to its natural anticoagulant functions, APC conveys multiple direct cytoprotective effects on many different cell types that involve multiple receptors including protease activated receptor (PAR) 1, PAR3, and the endothelial protein C receptor (EPCR). Application of molecular engineered APC variants with altered selectivity profiles to rodent stroke models demonstrated that the beneficial effects of APC primarily require its cytoprotective activities but not its anticoagulant activities. Extensive basic, preclinical, and clinical research provided a compelling rationale based on strong evidence for translation of APC therapy that has led to the clinical development of the cytoprotective-selective APC variant, 3K3A-APC, for ischaemic stroke. Recent identification of non-canonical PAR1 and PAR3 activation by APC that give rise to novel tethered-ligands capable of inducing biased cytoprotective signalling as opposed to the canonical signalling provides a mechanistic explanation for how APC-mediated PAR activation can selectively induce cytoprotective signalling pathways. Collectively, these paradigm-shifting discoveries provide detailed insights into the receptor targets and the molecular mechanisms for neuroprotection by cytoprotective-selective 3K3A-APC, which is currently a biologic drug in clinical trials for ischaemic stroke.
Keywords: EPCR; PAR1; PAR3; Stroke; activated protein C.
Conflict of interest statement
LO Mosnier, JH Griffin, and BV Zlokovic are inventors for subject matters related to cytoprotective, neuroprotective APC variants. JH Griffin is a consultant for ZZBiotech LLC. BV Zlokovic is the scientific founder of ZZBiotech LLC, a biotechnology company with a focus to develop activated protein C and its functional mutants for stroke and other neurological disorders.
Figures




Similar articles
-
Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3.Blood. 2013 Aug 1;122(5):807-16. doi: 10.1182/blood-2013-03-488957. Epub 2013 Jun 20. Blood. 2013. PMID: 23788139 Free PMC article.
-
2016 Scientific Sessions Sol Sherry Distinguished Lecturer in Thrombosis: Thrombotic Stroke: Neuroprotective Therapy by Recombinant-Activated Protein C.Arterioscler Thromb Vasc Biol. 2016 Nov;36(11):2143-2151. doi: 10.1161/ATVBAHA.116.308038. Epub 2016 Oct 6. Arterioscler Thromb Vasc Biol. 2016. PMID: 27758767 Free PMC article. Review.
-
A novel protein C-factor VII chimera provides new insights into the structural requirements for cytoprotective protease-activated receptor 1 signaling.J Thromb Haemost. 2017 Nov;15(11):2198-2207. doi: 10.1111/jth.13807. Epub 2017 Sep 21. J Thromb Haemost. 2017. PMID: 28834159
-
Activated protein C analog promotes neurogenesis and improves neurological outcome after focal ischemic stroke in mice via protease activated receptor 1.Brain Res. 2013 Apr 24;1507:97-104. doi: 10.1016/j.brainres.2013.02.023. Epub 2013 Feb 21. Brain Res. 2013. PMID: 23438513 Free PMC article.
-
Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway.J Thromb Haemost. 2013 Jun;11 Suppl 1(0 1):242-53. doi: 10.1111/jth.12247. J Thromb Haemost. 2013. PMID: 23809128 Free PMC article. Review.
Cited by
-
Evaluation of 3K3A-Activated Protein C to Treat Neonatal Hypoxic Ischemic Brain Injury in the Spiny Mouse.Neurotherapeutics. 2019 Jan;16(1):231-243. doi: 10.1007/s13311-018-0661-0. Neurotherapeutics. 2019. PMID: 30225791 Free PMC article.
-
Endothelial microparticles released by activated protein C protect beta cells through EPCR/PAR1 and annexin A1/FPR2 pathways in islets.J Cell Mol Med. 2017 Nov;21(11):2759-2772. doi: 10.1111/jcmm.13191. Epub 2017 May 19. J Cell Mol Med. 2017. PMID: 28524456 Free PMC article.
-
Safety and tolerability of the protein C activator AB002 in end-stage renal disease patients on hemodialysis: a randomized phase 2 trial.Commun Med (Lond). 2024 Jul 26;4(1):153. doi: 10.1038/s43856-024-00575-y. Commun Med (Lond). 2024. PMID: 39060370 Free PMC article.
-
Protease-Activated Receptor-1 Supports Locomotor Recovery by Biased Agonist Activated Protein C after Contusive Spinal Cord Injury.PLoS One. 2017 Jan 25;12(1):e0170512. doi: 10.1371/journal.pone.0170512. eCollection 2017. PLoS One. 2017. PMID: 28122028 Free PMC article.
-
Thrombin regulation of synaptic transmission and plasticity: implications for health and disease.Front Cell Neurosci. 2015 Apr 21;9:151. doi: 10.3389/fncel.2015.00151. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25954157 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

