Drift of the HIV-1 envelope glycoprotein gp120 toward increased neutralization resistance over the course of the epidemic: a comprehensive study using the most potent and broadly neutralizing monoclonal antibodies
- PMID: 25231299
- PMCID: PMC4248973
- DOI: 10.1128/JVI.02083-14
Drift of the HIV-1 envelope glycoprotein gp120 toward increased neutralization resistance over the course of the epidemic: a comprehensive study using the most potent and broadly neutralizing monoclonal antibodies
Abstract
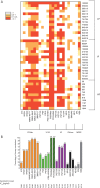
Extending our previous analyses to the most recently described monoclonal broadly neutralizing antibodies (bNAbs), we confirmed a drift of HIV-1 clade B variants over 2 decades toward higher resistance to bNAbs targeting almost all the identified gp120-neutralizing epitopes. In contrast, the sensitivity to bNAbs targeting the gp41 membrane-proximal external region remained stable, suggesting a selective pressure on gp120 preferentially. Despite this evolution, selected combinations of bNAbs remain capable of neutralizing efficiently most of the circulating variants.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 20 August 2014. Impact of clade, geography and age of the epidemic on HIV-1 neutralization by antibodies. J. Virol. 10.1128/JVI.01705-14. - DOI - PMC - PubMed
-
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 6:e1001028. 10.1371/journal.ppat.1001028. - DOI - PMC - PubMed
-
- Braibant M, Brunet S, Costagliola D, Rouzioux C, Agut H, Katinger H, Autran B, Barin F. 2006. Antibodies to conserved epitopes of the HIV-1 envelope in sera from long-term non-progressors: prevalence and association with neutralizing activity. AIDS 20:1923–1930. 10.1097/01.aids.0000247113.43714.5e. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

