Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis
- PMID: 25231318
- PMCID: PMC4248957
- DOI: 10.1128/JVI.02166-14
Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis
Abstract
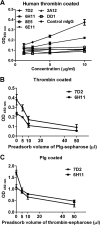
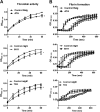
Dengue virus (DENV) is the most common cause of viral hemorrhagic fever, and it may lead to life-threating dengue hemorrhagic fever and shock syndrome (DHF/DSS). Because most cases of DHF/DSS occur in patients with secondary DENV infection, anti-DENV antibodies are generally considered to play a role in the pathogenesis of DHF/DSS. Previously, we have found that antithrombin antibodies (ATAs) with both antithrombotic and profibrinolytic activities are present in the sera of dengue patients. However, the mechanism by which these autoantibodies are induced is unclear. In this study, we demonstrated that antibodies induced by DENV immunization in mice and rabbits could bind to DENV antigens as well as to human thrombin and plasminogen (Plg). The binding of anti-DENV antibodies to thrombin and Plg was inhibited by preadsorption with DENV nonstructural protein 1. In addition, affinity-purified ATAs from DENV-immunized rabbit sera could inhibit thrombin activity and enhance Plg activation both in vitro and in vivo. Taken together, our results suggest that molecular mimicry between DENV and coagulation factors can induce the production of autoantibodies with biological effects similar to those of ATAs found in dengue patients. These coagulation-factor cross-reactive anti-DENV antibodies can interfere with the balance of coagulation and fibrinolysis, which may lead to the tendency of DHF/DSS patients to bleed.
Importance: Dengue virus (DENV) infection is the most common mosquito-borne viral disease in tropical and subtropical areas. Over 50 million DENV infection cases develop each year, and more than 2.5 billion people are at risk of dengue-induced hemorrhagic fever and shock syndrome. Currently, there is no vaccine or drug treatment for DENV. In the present study, we demonstrated that DENV immunization could induce thrombin and plasminogen (Plg) cross-reactive antibodies, which were able to inhibit thrombin activity and enhance Plg activation. These results suggest that molecular mimicry between DENV antigens, thrombin, and Plg may elicit antibodies that disturb hemostasis. The selection of appropriate candidate antigens for use in DENV vaccines should prevent these potentially dangerous autoimmune responses.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376–381. 10.1128/JCM.40.02.376-381.2002. - DOI - PMC - PubMed
-
- Halstead SB. 1982. Dengue: hematologic aspects. Semin. Hematol. 19:116–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

