GABA in nucleus tractus solitarius participates in electroacupuncture modulation of cardiopulmonary bradycardia reflex
- PMID: 25231352
- PMCID: PMC4254943
- DOI: 10.1152/ajpregu.00300.2014
GABA in nucleus tractus solitarius participates in electroacupuncture modulation of cardiopulmonary bradycardia reflex
Abstract
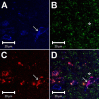
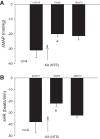
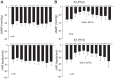
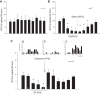
Phenylbiguanide (PBG) stimulates cardiopulmonary receptors and cardiovascular reflex responses, including decreases in blood pressure and heart rate mediated by the brain stem parasympathetic cardiac neurons in the nucleus ambiguus and nucleus tractus solitarius (NTS). Electroacupuncture (EA) at P5-6 stimulates sensory fibers in the median nerve and modulates these reflex responses. Stimulation of median nerves reverses bradycardia through action of γ-aminobutyric acid (GABA) in the nucleus ambiguus, important in the regulation of heart rate. We do not know whether the NTS or the neurotransmitter mechanisms in this nucleus participate in these modulatory actions by acupuncture. We hypothesized that somatic nerve stimulation during EA (P5-6) modulates cardiopulmonary inhibitory responses through a GABAergic mechanism in the NTS. Anesthetized and ventilated cats were examined during either PBG or direct vagal afferent stimulation while 30 min of EA was applied at P5-6. Reflex heart rate and blood pressure responses and NTS-evoked discharge were recorded. EA reduced the PBG-induced depressor and bradycardia reflexes by 67% and 60%, respectively. Blockade of GABAA receptors in the NTS reversed EA modulation of bradycardia but not the depressor response. During EA, gabazine reversed the vagally evoked discharge activity of cardiovascular NTS neurons. EA modulated the vagal-evoked cardiovascular NTS cellular activity for 60 min. Immunohistochemistry using triple labeling showed GABA immunoreactive fibers juxtaposed to glutamatergic nucleus ambiguus-projecting NTS neurons in rats. These glutamatergic neurons expressed GABAA receptors. These findings suggest that EA inhibits PBG-evoked bradycardia and vagally evoked NTS activity through a GABAergic mechanism, likely involving glutamatergic nucleus ambiguus-projecting NTS neurons.
Keywords: glutamic acid; phenylbiguanide; vagal excitation.
Copyright © 2014 the American Physiological Society.
Figures




Similar articles
-
Modulation of cardiopulmonary depressor reflex in nucleus ambiguus by electroacupuncture: roles of opioids and γ-aminobutyric acid.Am J Physiol Regul Integr Comp Physiol. 2012 Apr;302(7):R833-44. doi: 10.1152/ajpregu.00440.2011. Epub 2011 Dec 28. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22204951 Free PMC article.
-
Modulation of Neurally Mediated Vasodepression and Bradycardia by Electroacupuncture through Opioids in Nucleus Tractus Solitarius.Sci Rep. 2018 Jan 30;8(1):1900. doi: 10.1038/s41598-018-19672-9. Sci Rep. 2018. PMID: 29382866 Free PMC article.
-
Medullary GABAergic mechanisms contribute to electroacupuncture modulation of cardiovascular depressor responses during gastric distention in rats.Am J Physiol Regul Integr Comp Physiol. 2013 Mar 1;304(5):R321-32. doi: 10.1152/ajpregu.00451.2012. Epub 2013 Jan 9. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23302958 Free PMC article.
-
Short-term receptor trafficking in the dorsal vagal complex: an overview.Auton Neurosci. 2006 Jun 30;126-127:2-8. doi: 10.1016/j.autneu.2006.01.019. Epub 2006 Mar 6. Auton Neurosci. 2006. PMID: 16580267 Free PMC article. Review.
-
Glutamatergic neurons say NO in the nucleus tractus solitarii.J Chem Neuroanat. 2009 Nov;38(3):154-65. doi: 10.1016/j.jchemneu.2009.02.002. Epub 2009 Feb 21. J Chem Neuroanat. 2009. PMID: 19778681 Free PMC article. Review.
Cited by
-
Effect of GABA on blood pressure and blood dynamics of anesthetic rats.Int J Clin Exp Med. 2015 Aug 15;8(8):14296-302. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26550413 Free PMC article.
-
Neurophysiological Basis of Electroacupuncture Stimulation in the Treatment of Cardiovascular-Related Diseases: Vagal Interoceptive Loops.Brain Behav. 2024 Oct;14(10):e70076. doi: 10.1002/brb3.70076. Brain Behav. 2024. PMID: 39344397 Free PMC article. Review.
-
Effect of acupuncture on hormone level in patients with gastrointestinal dysfunction after general anesthesia: A study protocol for a randomized controlled trial.Medicine (Baltimore). 2020 Apr;99(14):e19610. doi: 10.1097/MD.0000000000019610. Medicine (Baltimore). 2020. PMID: 32243385 Free PMC article.
-
Acupuncture activates a direct pathway from the nucleus tractus solitarii to the rostral ventrolateral medulla.Brain Res. 2019 Apr 1;1708:69-77. doi: 10.1016/j.brainres.2018.12.009. Epub 2018 Dec 6. Brain Res. 2019. PMID: 30529283 Free PMC article.
-
Paraventricular Nucleus Modulates Excitatory Cardiovascular Reflexes during Electroacupuncture.Sci Rep. 2016 May 16;6:25910. doi: 10.1038/srep25910. Sci Rep. 2016. PMID: 27181844 Free PMC article.
References
-
- Aviado DM, Guevara AD. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann NY Acad Sci 940: 48–58, 2001. - PubMed
-
- Berman AL. The Brainstem of the Cat: A Cytoarchitectonic Atlas With Stereotaxic Coordinates. Madison, WI: The University of Wisconsin Press, 1968.
-
- Chao DM, Shen LL, Tjen-ALooi SC, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127–H2134, 1999. - PubMed
-
- Cheung L, Li P, Wong C. The Mechanism of Acupuncture Therapy and Clinical Case Studies. New York: Taylor and Francis, 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

