The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial
- PMID: 25231491
- PMCID: PMC4166247
- DOI: 10.1136/bmjopen-2014-005651
The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial
Abstract
Introduction: Postoperative delirium is one of the most common complications of major surgery, affecting 10-70% of surgical patients 60 years and older. Delirium is an acute change in cognition that manifests as poor attention and illogical thinking and is associated with longer intensive care unit (ICU) and hospital stay, long-lasting cognitive deterioration and increased mortality. Ketamine has been used as an anaesthetic drug for over 50 years and has an established safety record. Recent research suggests that, in addition to preventing acute postoperative pain, a subanaesthetic dose of intraoperative ketamine could decrease the incidence of postoperative delirium as well as other neurological and psychiatric outcomes. However, these proposed benefits of ketamine have not been tested in a large clinical trial.
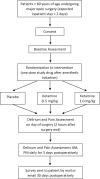
Methods: The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study is an international, multicentre, randomised controlled trial. 600 cardiac and major non-cardiac surgery patients will be randomised to receive ketamine (0.5 or 1 mg/kg) or placebo following anaesthetic induction and prior to surgical incision. For the primary outcome, blinded observers will assess delirium on the day of surgery (postoperative day 0) and twice daily from postoperative days 1-3 using the Confusion Assessment Method or the Confusion Assessment Method for the ICU. For the secondary outcomes, blinded observers will estimate pain using the Behavioral Pain Scale or the Behavioral Pain Scale for Non-Intubated Patients and patient self-report.
Ethics and dissemination: The PODCAST trial has been approved by the ethics boards of five participating institutions; approval is ongoing at other sites. Recruitment began in February 2014 and will continue until the end of 2016. Dissemination plans include presentations at scientific conferences, scientific publications, stakeholder engagement and popular media.
Registration details: The study is registered at clinicaltrials.gov, NCT01690988 (last updated March 2014). The PODCAST trial is being conducted under the auspices of the Neurological Outcomes Network for Surgery (NEURONS).
Trial registration number: NCT01690988 (last updated December 2013).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
References
-
- Koster S, Hensens AG, Schuurmans MJ, et al. Consequences of delirium after cardiac operations. Ann Thorac Surg 2012;93:705–11 - PubMed
-
- Kat MG, Vreeswijk R, de Jonghe JF, et al. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord 2008;26:1–8 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical