Genetic Analysis of Diffuse High-Grade Astrocytomas in Infancy Defines a Novel Molecular Entity
- PMID: 25231549
- PMCID: PMC8029085
- DOI: 10.1111/bpa.12210
Genetic Analysis of Diffuse High-Grade Astrocytomas in Infancy Defines a Novel Molecular Entity
Abstract
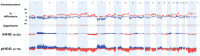
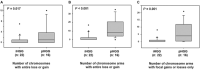
Pediatric high-grade gliomas are considered to be different when compared to adult high-grade gliomas in their pathogenesis and biological behavior. Recently, common genetic alterations, including mutations in the H3F3A/ATRX/DAXX pathway, have been described in approximately 30% of the pediatric cases. However, only few cases of infant high-grade gliomas have been analyzed so far. We investigated the molecular features of 35 infants with diffuse high-grade astrocytomas, including 8 anaplastic astrocytomas [World Health Organization (WHO) grade III] and 27 glioblastomas (WHO grade IV) by immunohistochemistry, multiplex ligation probe-dependent amplification (MLPA), pyrosequencing of glioma-associated genes and molecular inversion probe (MIP) assay. MIP and MLPA analyses showed that chromosomal alterations are significantly less frequent in infants compared with high-grade gliomas in older children and adults. We only identified H3F3A K27M in 2 of 34 cases (5.9%), with both tumors located in the posterior fossa. PDGFRA amplifications were absent, and CDKN2A loss could be observed only in two cases. Conversely, 1q gain (22.7%) and 6q loss (18.2%) were identified in a subgroup of tumors. Loss of SNORD located on chromosome 14q32 was observed in 27.3% of the infant tumors, a focal copy number change not previously described in gliomas. Our findings indicate that infant high-grade gliomas appear to represent a distinct genetic entity suggesting a different pathogenesis and biological behavior.
Keywords: ALT; ATRX; H3F3A; SNORD; glioblastoma; high-grade glioma; infants; molecular inversion probe analysis; multiplex ligation probe-dependent amplification; pediatric brain tumors.
© 2014 International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Abedalthagafi M, Phillips JJ, Kim GE, Mueller S, Haas‐Kogen DA, Marshall RE et al (2013) The alternative lengthening of telomere phenotype is significantly associated with loss of ATRX expression in high‐grade pediatric and adult astrocytomas: a multi‐institutional study of 214 astrocytomas. Mod Pathol 26:1425–1432. - PMC - PubMed
-
- Antonelli M, Buttarelli FR, Arcella A, Nobusawa S, Donofrio V, Oghaki H, Giangaspero F (2010) Prognostic significance of histological grading, p53 status, YKL‐40 expression, and IDH1 mutations in pediatric high‐grade gliomas. J Neurooncol 5:209–215. - PubMed
-
- Batra V, Sands SA, Holmes E, Russell Geyer J, Yates A, Becker L et al (2014) Long‐term survival of children less than six years of age enrolled on the CCG‐945 phase III trial for newly‐diagnosed high‐grade glioma: a report from the Children's Oncology Group. Pediatr Blood Cancer 61:151–157. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

