Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice
- PMID: 25231679
- PMCID: PMC5377307
- DOI: 10.1038/srep06370
Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice
Abstract
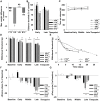
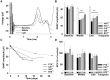
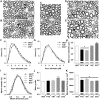
Polyneuropathy is a frequent and potentially severe side effect of clinical tumor chemotherapy. The goal of this study was to characterize paclitaxel-, cisplatin-, vincristine- and bortezomib-induced neuropathy in C57BL/6 mice with a comparative approach. The phenotype of the animals was evaluated at four time points with behavioral and electrophysiological tests, followed by histology. Treatment protocols used in this study were well tolerated and induced a sensory and predominantly axonal polyneuropathy. Behavioral testing revealed normal motor coordination, whereas all mice receiving verum treatment developed mechanical allodynia and distinct gait alterations. Electrophysiological evaluation showed a significant decrease of the caudal sensory nerve action potential amplitude for all cytostatic agents and a moderate reduction of nerve conduction velocity for cisplatin and paclitaxel. This finding was confirmed by histological analysis of the sciatic nerve which showed predominantly axonal damage: Paclitaxel and vincristine affected mostly large myelinated fibers, bortezomib small myelinated fibers and cisplatin damaged all types of myelinated fibers to a similar degree. Neuropathic symptoms developed faster in paclitaxel and vincristine treated animals compared to cisplatin and bortezomib treatment. The animal models in this study can be used to elucidate pathomechanisms underlying chemotherapy-induced polyneuropathy and for the development of novel therapeutic and preventative strategies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Heterozygous P0 deficiency protects mice from vincristine-induced polyneuropathy.J Neurosci Res. 2006 Jul;84(1):37-46. doi: 10.1002/jnr.20873. J Neurosci Res. 2006. PMID: 16676325
-
Neurophysiological, histological and immunohistochemical characterization of bortezomib-induced neuropathy in mice.Exp Neurol. 2010 Jun;223(2):599-608. doi: 10.1016/j.expneurol.2010.02.006. Epub 2010 Feb 24. Exp Neurol. 2010. PMID: 20188093
-
Assessment of paclitaxel induced sensory polyneuropathy with "Catwalk" automated gait analysis in mice.PLoS One. 2013 Oct 15;8(10):e76772. doi: 10.1371/journal.pone.0076772. eCollection 2013. PLoS One. 2013. PMID: 24143194 Free PMC article.
-
Rodent models of chemotherapy-induced peripheral neuropathy.ILAR J. 2014;54(3):273-81. doi: 10.1093/ilar/ilt053. ILAR J. 2014. PMID: 24615440 Review.
-
Vincristine- and bortezomib-induced neuropathies - from bedside to bench and back.Exp Neurol. 2021 Feb;336:113519. doi: 10.1016/j.expneurol.2020.113519. Epub 2020 Oct 29. Exp Neurol. 2021. PMID: 33129841 Free PMC article. Review.
Cited by
-
The impact of SBF2 on taxane-induced peripheral neuropathy.PLoS Genet. 2022 Jan 5;18(1):e1009968. doi: 10.1371/journal.pgen.1009968. eCollection 2022 Jan. PLoS Genet. 2022. PMID: 34986146 Free PMC article.
-
Minocycline Prevents the Development of Mechanical Allodynia in Mouse Models of Vincristine-Induced Peripheral Neuropathy.Front Neurosci. 2019 Jun 27;13:653. doi: 10.3389/fnins.2019.00653. eCollection 2019. Front Neurosci. 2019. PMID: 31316337 Free PMC article.
-
Solute Carrier Transportome in Chemotherapy-Induced Adverse Drug Reactions.Rev Physiol Biochem Pharmacol. 2022;183:177-215. doi: 10.1007/112_2020_30. Rev Physiol Biochem Pharmacol. 2022. PMID: 32761456 Free PMC article.
-
Evaluation of the Therapeutic Potential of Amantadine in a Vincristine-Induced Peripheral Neuropathy Model in Rats.Animals (Basel). 2024 Jun 30;14(13):1941. doi: 10.3390/ani14131941. Animals (Basel). 2024. PMID: 38998053 Free PMC article.
-
Suramin-Induced Neurotoxicity: Preclinical Models and Neuroprotective Strategies.Molecules. 2018 Feb 7;23(2):346. doi: 10.3390/molecules23020346. Molecules. 2018. PMID: 29414872 Free PMC article.
References
-
- Windebank A. J. & Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 13, 27–46 (2008). - PubMed
-
- Schiff P. B., Fant J. & Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667 (1979). - PubMed
-
- Johnson I. S., Armstrong J. G., Gorman M. & Burnett J. P. Jr The Vinca Alkaloids: A New Class of Oncolytic Agents. Cancer Res 23, 1390–1427 (1963). - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7, 573–584 (2007). - PubMed
-
- Mohty M., Brissot E., Savani B. N. & Gaugler B. Effects of bortezomib on the immune system: a focus on immune regulation. BBMT 19, 1416–1420 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

