Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†
- PMID: 25231953
- PMCID: PMC6267855
- DOI: 10.1093/annonc/mdu456
Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†
Abstract
Background: The BOLERO-2 study previously demonstrated that adding everolimus (EVE) to exemestane (EXE) significantly improved progression-free survival (PFS) by more than twofold in patients with hormone-receptor-positive (HR(+)), HER2-negative advanced breast cancer that recurred or progressed during/after treatment with nonsteroidal aromatase inhibitors (NSAIs). The overall survival (OS) analysis is presented here.
Patients and methods: BOLERO-2 is a phase III, double-blind, randomized international trial comparing EVE 10 mg/day plus EXE 25 mg/day versus placebo (PBO) + EXE 25 mg/day in postmenopausal women with HR(+) advanced breast cancer with prior exposure to NSAIs. The primary end point was PFS by local investigator assessment; OS was a key secondary end point.
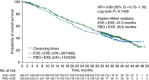
Results: At the time of data cutoff (3 October 2013), 410 deaths had occurred and 13 patients remained on treatment. Median OS in patients receiving EVE + EXE was 31.0 months [95% confidence interval (CI) 28.0-34.6 months] compared with 26.6 months (95% CI 22.6-33.1 months) in patients receiving PBO + EXE (hazard ratio = 0.89; 95% CI 0.73-1.10; log-rank P = 0.14). Poststudy treatments were received by 84% of patients in the EVE + EXE arm versus 90% of patients in the PBO + EXE arm. Types of poststudy therapies were balanced across arms, except for chemotherapy (53% EVE + EXE versus 63% PBO + EXE). No new safety concerns were identified.
Conclusions: In BOLERO-2, adding EVE to EXE did not confer a statistically significant improvement in the secondary end point OS despite producing a clinically meaningful and statistically significant improvement in the primary end point, PFS (4.6-months prolongation in median PFS; P < 0.0001). Ongoing translational research should further refine the benefit of mTOR inhibition and related pathways in this treatment setting.
Trial registration number: NCT00863655.
Keywords: everolimus; exemestane; hormone-receptor-positive breast cancer; overall survival.
© The Author 2014. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, v2.2014. www.nccn.org. 3 April 2014, date last accessed. - PubMed
-
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21:242–252. - PubMed
-
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. - PubMed
-
- Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. - PubMed
-
- Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14:989–998. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous