MicroRNA-155 potentiates the inflammatory response in hypothermia by suppressing IL-10 production
- PMID: 25231976
- PMCID: PMC4232280
- DOI: 10.1096/fj.14-258335
MicroRNA-155 potentiates the inflammatory response in hypothermia by suppressing IL-10 production
Abstract
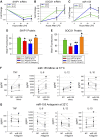
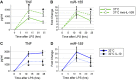
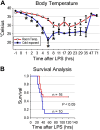
Therapeutic hypothermia is commonly used to improve neurological outcomes in patients after cardiac arrest. However, therapeutic hypothermia increases sepsis risk and unintentional hypothermia in surgical patients increases infectious complications. Nonetheless, the molecular mechanisms by which hypothermia dysregulates innate immunity are incompletely understood. We found that exposure of human monocytes to cold (32°C) potentiated LPS-induced production of TNF and IL-6, while blunting IL-10 production. This dysregulation was associated with increased expression of microRNA-155 (miR-155), which potentiates Toll-like receptor (TLR) signaling by negatively regulating Ship1 and Socs1. Indeed, Ship1 and Socs1 were suppressed at 32°C and miR-155 antagomirs increased Ship1 and Socs1 and reversed the alterations in cytokine production in cold-exposed monocytes. In contrast, miR-155 mimics phenocopied the effects of cold exposure, reducing Ship1 and Socs1 and altering TNF and IL-10 production. In a murine model of LPS-induced peritonitis, cold exposure potentiated hypothermia and decreased survival (10 vs. 50%; P < 0.05), effects that were associated with increased miR-155, suppression of Ship1 and Socs1, and alterations in TNF and IL-10. Importantly, miR-155-deficiency reduced hypothermia and improved survival (78 vs. 32%, P < 0.05), which was associated with increased Ship1, Socs1, and IL-10. These results establish a causal role of miR-155 in the dysregulation of the inflammatory response to hypothermia.
Keywords: cold exposure; peritonitis; sepsis.
© FASEB.
Figures





References
-
- Arrich J., Holzer M., Havel C., Mullner M., Herkner H. (2012) Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 9, CD004128. - PubMed
-
- Matsui T., Yoshida Y., Yanagihara M., Suenaga H. (2014) Hypothermia at 35 degrees C reduces the time-dependent microglial production of pro-inflammatory and anti-inflammatory factors that mediate neuronal cell death. Neurocrit. Care 20, 301–310 - PubMed
-
- Tian D. H., Wan B., Bannon P. G., Misfeld M., Lemaire S. A., Kazui T., Kouchoukos N. T., Elefteriades J. A., Bavaria J., Coselli J. S., Griepp R. B., Mohr F. W., Oo A., Svensson L. G., Hughes G. C., Yan T. D. (2013) A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2, 148–158 - PMC - PubMed
-
- Ahmad F. U., Wang M. Y., Levi A. D. (2014) Hypothermia for acute spinal cord injury–a review. World Neurosurg. 82, 207–214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

