Interplay between sumoylation and phosphorylation for protection against α-synuclein inclusions
- PMID: 25231978
- PMCID: PMC4223324
- DOI: 10.1074/jbc.M114.559237
Interplay between sumoylation and phosphorylation for protection against α-synuclein inclusions
Abstract
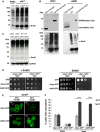
Parkinson disease is associated with the progressive loss of dopaminergic neurons from the substantia nigra. The pathological hallmark of the disease is the accumulation of intracytoplasmic inclusions known as Lewy bodies that consist mainly of post-translationally modified forms of α-synuclein. Whereas phosphorylation is one of the major modifications of α-synuclein in Lewy bodies, sumoylation has recently been described. The interplay between α-synuclein phosphorylation and sumoylation is poorly understood. Here, we examined the interplay between these modifications as well as their impact on cell growth and inclusion formation in yeast. We found that α-synuclein is sumoylated in vivo at the same sites in yeast as in human cells. Impaired sumoylation resulted in reduced yeast growth combined with an increased number of cells with inclusions, suggesting that this modification plays a protective role. In addition, inhibition of sumoylation prevented autophagy-mediated aggregate clearance. A defect in α-synuclein sumoylation could be suppressed by serine 129 phosphorylation by the human G protein-coupled receptor kinase 5 (GRK5) in yeast. Phosphorylation reduced foci formation, alleviated yeast growth inhibition, and partially rescued autophagic α-synuclein degradation along with the promotion of proteasomal degradation, resulting in aggregate clearance in the absence of a small ubiquitin-like modifier. These findings suggest a complex interplay between sumoylation and phosphorylation in α-synuclein aggregate clearance, which may open new horizons for the development of therapeutic strategies for Parkinson disease.
Keywords: Autophagy; Post-translational Modification; Proteasome; Yeast; α-Synuclein.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures










References
-
- Obeso J. A., Rodriguez-Oroz M. C., Goetz C. G., Marin C., Kordower J. H., Rodriguez M., Hirsch E. C., Farrer M., Schapira A. H., Halliday G. (2010) Missing pieces in the Parkinson's disease puzzle. Nat. Med. 16, 653–661 - PubMed
-
- Galvin J. E., Lee V. M., Trojanowski J. Q. (2001) Synucleinopathies: clinical and pathological implications. Arch. Neurol. 58, 186–190 - PubMed
-
- Forno L. S., DeLanney L. E., Irwin I., Langston J. W. (1996) Electron microscopy of Lewy bodies in the amygdala-parahippocampal region. Comparison with inclusion bodies in the MPTP-treated squirrel monkey. Adv. Neurol. 69, 217–228 - PubMed
-
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 - PubMed
-
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

