Crystal structure of a lipoxygenase in complex with substrate: the arachidonic acid-binding site of 8R-lipoxygenase
- PMID: 25231982
- PMCID: PMC4231669
- DOI: 10.1074/jbc.M114.599662
Crystal structure of a lipoxygenase in complex with substrate: the arachidonic acid-binding site of 8R-lipoxygenase
Abstract
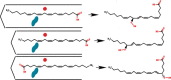
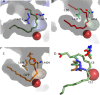
Lipoxygenases (LOX) play critical roles in mammalian biology in the generation of potent lipid mediators of the inflammatory response; consequently, they are targets for the development of isoform-specific inhibitors. The regio- and stereo-specificity of the oxygenation of polyunsaturated fatty acids by the enzymes is understood in terms of the chemistry, but structural observation of the enzyme-substrate interactions is lacking. Although several LOX crystal structures are available, heretofore the rapid oxygenation of bound substrate has precluded capture of the enzyme-substrate complex, leaving a gap between chemical and structural insights. In this report, we describe the 2.0 Å resolution structure of 8R-LOX in complex with arachidonic acid obtained under anaerobic conditions. Subtle rearrangements, primarily in the side chains of three amino acids, allow binding of arachidonic acid in a catalytically competent conformation. Accompanying experimental work supports a model in which both substrate tethering and cavity depth contribute to positioning the appropriate carbon at the catalytic machinery.
Keywords: Arachidonic Acid (AA) (ARA); Eicosanoid Biosynthesis; Lipid Signaling; Lipoxygenase Pathway; Protein Structure; X-ray Crystallography.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Yamamoto S. (1992) Mammalian lipoxygenases: molecular structures and functions. Biochim. Biophys. Acta 1128, 117–131 - PubMed
-
- Brash A. R. (1999) Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274, 23679–23682 - PubMed
-
- Ivanov I., Heydeck D., Hofheinz K., Roffeis J., O'Donnell V. B., Kuhn H., Walther M. (2010) Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 503, 161–174 - PubMed
-
- Andreou A., Feussner I. (2009) Lipoxygenases: structure and reaction mechanism. Phytochemistry 70, 1504–1510 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

