Translational roles of elongation factor 2 protein lysine methylation
- PMID: 25231983
- PMCID: PMC4215232
- DOI: 10.1074/jbc.M114.605527
Translational roles of elongation factor 2 protein lysine methylation
Abstract
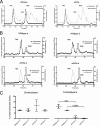
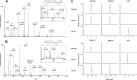
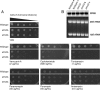
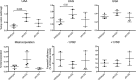
Methylation of various components of the translational machinery has been shown to globally affect protein synthesis. Little is currently known about the role of lysine methylation on elongation factors. Here we show that in Saccharomyces cerevisiae, the product of the EFM3/YJR129C gene is responsible for the trimethylation of lysine 509 on elongation factor 2. Deletion of EFM3 or of the previously described EFM2 increases sensitivity to antibiotics that target translation and decreases translational fidelity. Furthermore, the amino acid sequences of Efm3 and Efm2, as well as their respective methylation sites on EF2, are conserved in other eukaryotes. These results suggest the importance of lysine methylation modification of EF2 in fine tuning the translational apparatus.
Keywords: Lysine Methylation; Protein Methylation; Protein Synthesis; Ribosome Function; S-Adenosylmethionine (SAM); Translation Elongation Factor.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Elongation factor methyltransferase 3--a novel eukaryotic lysine methyltransferase.Biochem Biophys Res Commun. 2014 Aug 22;451(2):229-34. doi: 10.1016/j.bbrc.2014.07.110. Epub 2014 Jul 30. Biochem Biophys Res Commun. 2014. PMID: 25086354
-
Identification and characterization of a novel evolutionarily conserved lysine-specific methyltransferase targeting eukaryotic translation elongation factor 2 (eEF2).J Biol Chem. 2014 Oct 31;289(44):30499-30510. doi: 10.1074/jbc.M114.601658. Epub 2014 Sep 17. J Biol Chem. 2014. PMID: 25231979 Free PMC article.
-
Stoichiometry of Saccharomyces cerevisiae lysine methylation: insights into non-histone protein lysine methyltransferase activity.J Proteome Res. 2014 Mar 7;13(3):1744-56. doi: 10.1021/pr401251k. Epub 2014 Feb 21. J Proteome Res. 2014. PMID: 24517342
-
The diphthamide modification pathway from Saccharomyces cerevisiae--revisited.Mol Microbiol. 2014 Dec;94(6):1213-26. doi: 10.1111/mmi.12845. Epub 2014 Nov 17. Mol Microbiol. 2014. PMID: 25352115 Review.
-
Using Yeast to Define the Regulatory Role of Protein Lysine Methylation.Curr Protein Pept Sci. 2020;21(7):690-698. doi: 10.2174/1389203720666191023150727. Curr Protein Pept Sci. 2020. PMID: 31642774 Free PMC article. Review.
Cited by
-
Evolutionary insights into Trm112-methyltransferase holoenzymes involved in translation between archaea and eukaryotes.Nucleic Acids Res. 2018 Sep 19;46(16):8483-8499. doi: 10.1093/nar/gky638. Nucleic Acids Res. 2018. PMID: 30010922 Free PMC article.
-
Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae.Genetics. 2016 May;203(1):65-107. doi: 10.1534/genetics.115.186221. Genetics. 2016. PMID: 27183566 Free PMC article. Review.
-
Hydroxylation and translational adaptation to stress: some answers lie beyond the STOP codon.Cell Mol Life Sci. 2016 May;73(9):1881-93. doi: 10.1007/s00018-016-2160-y. Epub 2016 Feb 13. Cell Mol Life Sci. 2016. PMID: 26874685 Free PMC article. Review.
-
Lysine Methylation of the Valosin-Containing Protein (VCP) Is Dispensable for Development and Survival of Mice.PLoS One. 2015 Nov 6;10(11):e0141472. doi: 10.1371/journal.pone.0141472. eCollection 2015. PLoS One. 2015. PMID: 26544960 Free PMC article.
-
Novel N-terminal and Lysine Methyltransferases That Target Translation Elongation Factor 1A in Yeast and Human.Mol Cell Proteomics. 2016 Jan;15(1):164-76. doi: 10.1074/mcp.M115.052449. Epub 2015 Nov 6. Mol Cell Proteomics. 2016. PMID: 26545399 Free PMC article.
References
-
- Graille M., Figaro S., Kervestin S., Buckingham R. H., Liger D., Heurgué-Hamard V. (2012) Methylation of class I translation termination factors: structural and functional aspects. Biochimie 94, 1533–1543 - PubMed
-
- Liu J., Jia G. (2014) Methylation modifications in eukaryotic messenger RNA. J. Genet. Genomics 41, 21–33 - PubMed
-
- Motorin Y., Helm M. (2011) RNA nucleotide methylation. Wiley Interdiscip. Rev. RNA 2, 611–631 - PubMed
-
- Polevoda B., Sherman F. (2007) Methylation of proteins involved in translation. Mol. Microbiol. 65, 590–606 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

