Geminin inhibits a late step in the formation of human pre-replicative complexes
- PMID: 25231993
- PMCID: PMC4215257
- DOI: 10.1074/jbc.M114.552935
Geminin inhibits a late step in the formation of human pre-replicative complexes
Abstract
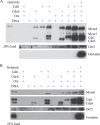
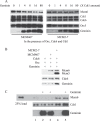
The initial step in initiation of eukaryotic DNA replication involves the assembly of pre-replicative complexes (pre-RCs) at origins of replication during the G1 phase of the cell cycle. In metazoans initiation is inhibited by the regulatory factor Geminin. We have purified the human pre-RC proteins, studied their interactions in vitro with each other and with origin DNA, and analyzed the effects of HsGeminin on formation of DNA-protein complexes. The formation of an initial complex containing the human origin recognition complex (HsORC), HsCdt1, HsCdc6, and origin DNA is cooperative, involving all possible binary interactions among the components. Maximal association of HsMCM2-7, a component of the replicative helicase, requires HsORC, HsCdc6, HsCdt1, and ATP, and is driven by interactions of HsCdt1 and HsCdc6 with multiple HsMCM2-7 subunits. Formation of stable complexes, resistant to high salt, requires ATP hydrolysis. In the absence of HsMCM proteins, HsGeminin inhibits the association of HsCdt1 with DNA or with HsORC-HsCdc6-DNA complexes. However, HsGeminin does not inhibit recruitment of HsMCM2-7 to DNA to form complexes containing all of the pre-RC proteins. In fact, HsGeminin itself is a component of such complexes, and interacts directly with the HsMcm3 and HsMcm5 subunits of HsMCM2-7, as well as with HsCdt1. Although HsGeminin does not prevent the initial formation of DNA-protein complexes containing the pre-RC proteins, it strongly inhibits the formation of stable pre-RCs that are resistant to high salt. We suggest that bound HsGeminin prevents transition of the pre-RC to a state that is competent for initiation of DNA replication.
Keywords: DNA Replication; DNA-Protein Interaction; Geminin; Pre-RC Assembly; Protein Assembly; Protein Complex; Protein-Protein Interaction.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Machida Y. J., Hamlin J. L., Dutta A. (2005) Right place, right time, and only once: replication initiation in metazoans. Cell 123, 13–24 - PubMed
-
- Neuwald A. F., Aravind L., Spouge J. L., Koonin E. V. (1999) AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

