A1 adenosine receptor-stimulated exocytosis in bladder umbrella cells requires phosphorylation of ADAM17 Ser-811 and EGF receptor transactivation
- PMID: 25232008
- PMCID: PMC4230785
- DOI: 10.1091/mbc.E14-03-0818
A1 adenosine receptor-stimulated exocytosis in bladder umbrella cells requires phosphorylation of ADAM17 Ser-811 and EGF receptor transactivation
Abstract
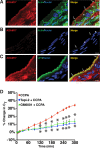
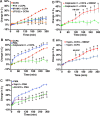
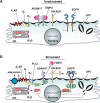
Despite the importance of ADAM17-dependent cleavage in normal biology and disease, the physiological cues that trigger its activity, the effector pathways that promote its function, and the mechanisms that control its activity, particularly the role of phosphorylation, remain unresolved. Using native bladder epithelium, in some cases transduced with adenoviruses encoding small interfering RNA, we observe that stimulation of apically localized A1 adenosine receptors (A1ARs) triggers a Gi-Gβγ-phospholipase C-protein kinase C (PKC) cascade that promotes ADAM17-dependent HB-EGF cleavage, EGFR transactivation, and apical exocytosis. We further show that the cytoplasmic tail of rat ADAM17 contains a conserved serine residue at position 811, which resides in a canonical PKC phosphorylation site, and is phosphorylated in response to A1AR activation. Preventing this phosphorylation event by expression of a nonphosphorylatable ADAM17(S811A) mutant or expression of a tail-minus construct inhibits A1AR-stimulated, ADAM17-dependent HB-EGF cleavage. Furthermore, expression of ADAM17(S811A) in bladder tissues impairs A1AR-induced apical exocytosis. We conclude that adenosine-stimulated exocytosis requires PKC- and ADAM17-dependent EGFR transactivation and that the function of ADAM17 in this pathway depends on the phosphorylation state of Ser-811 in its cytoplasmic domain.
© 2014 Prakasam et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




Similar articles
-
ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding.Invest Ophthalmol Vis Sci. 2009 Jan;50(1):132-9. doi: 10.1167/iovs.08-2246. Epub 2008 Jul 24. Invest Ophthalmol Vis Sci. 2009. PMID: 18658095 Free PMC article.
-
ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17.PLoS One. 2013;8(1):e54391. doi: 10.1371/journal.pone.0054391. Epub 2013 Jan 18. PLoS One. 2013. PMID: 23349873 Free PMC article.
-
HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells.Am J Physiol Renal Physiol. 2011 Apr;300(4):F921-31. doi: 10.1152/ajprenal.00436.2010. Epub 2011 Feb 2. Am J Physiol Renal Physiol. 2011. PMID: 21289053
-
ADAM17 participates in the protective effect of paeoniflorin on mouse brain microvascular endothelial cells.J Cell Physiol. 2018 Dec;233(12):9320-9329. doi: 10.1002/jcp.26308. Epub 2018 Jun 19. J Cell Physiol. 2018. PMID: 29215702
-
The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process.J Reprod Dev. 2012;58(5):510-4. doi: 10.1262/jrd.2012-056. J Reprod Dev. 2012. PMID: 23124701 Review.
Cited by
-
REG4 promotes peritoneal metastasis of gastric cancer through GPR37.Oncotarget. 2016 May 10;7(19):27874-88. doi: 10.18632/oncotarget.8442. Oncotarget. 2016. PMID: 27036049 Free PMC article.
-
Expression of Transgenes in Native Bladder Urothelium using Adenovirus-Mediated Transduction.J Vis Exp. 2022 Oct 6;(188):10.3791/64584. doi: 10.3791/64584. J Vis Exp. 2022. PMID: 36282713 Free PMC article.
-
Analysis of mutations in primary and metastatic synovial sarcoma.Oncotarget. 2018 Dec 7;9(96):36878-36888. doi: 10.18632/oncotarget.26416. eCollection 2018 Dec 7. Oncotarget. 2018. PMID: 30627328 Free PMC article.
-
Regulation of Fibrotic Processes in the Liver by ADAM Proteases.Cells. 2019 Oct 9;8(10):1226. doi: 10.3390/cells8101226. Cells. 2019. PMID: 31601007 Free PMC article. Review.
-
RAB27B requirement for stretch-induced exocytosis in bladder umbrella cells.Am J Physiol Cell Physiol. 2018 Mar 1;314(3):C349-C365. doi: 10.1152/ajpcell.00218.2017. Epub 2017 Nov 22. Am J Physiol Cell Physiol. 2018. PMID: 29167152 Free PMC article.
References
-
- Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knauper V, et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. - PubMed
-
- Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:1–12. - PubMed
-
- Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des. 2009;15:2319–2335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

