Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis
- PMID: 25232064
- PMCID: PMC4166201
- DOI: 10.1136/bmj.g5264
Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis
Abstract
Objective: To determine the accuracy of testing for human papillomavirus (HPV) DNA in urine in detecting cervical HPV in sexually active women.
Design: Systematic review and meta-analysis.
Data sources: Searches of electronic databases from inception until December 2013, checks of reference lists, manual searches of recent issues of relevant journals, and contact with experts.
Eligibility criteria: Test accuracy studies in sexually active women that compared detection of urine HPV DNA with detection of cervical HPV DNA.
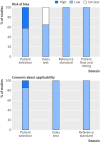
Data extraction and synthesis: Data relating to patient characteristics, study context, risk of bias, and test accuracy. 2 × 2 tables were constructed and synthesised by bivariate mixed effects meta-analysis.
Results: 16 articles reporting on 14 studies (1443 women) were eligible for meta-analysis. Most used commercial polymerase chain reaction methods on first void urine samples. Urine detection of any HPV had a pooled sensitivity of 87% (95% confidence interval 78% to 92%) and specificity of 94% (95% confidence interval 82% to 98%). Urine detection of high risk HPV had a pooled sensitivity of 77% (68% to 84%) and specificity of 88% (58% to 97%). Urine detection of HPV 16 and 18 had a pooled sensitivity of 73% (56% to 86%) and specificity of 98% (91% to 100%). Metaregression revealed an increase in sensitivity when urine samples were collected as first void compared with random or midstream (P=0.004).
Limitations: The major limitations of this review are the lack of a strictly uniform method for the detection of HPV in urine and the variation in accuracy between individual studies.
Conclusions: Testing urine for HPV seems to have good accuracy for the detection of cervical HPV, and testing first void urine samples is more accurate than random or midstream sampling. When cervical HPV detection is considered difficult in particular subgroups, urine testing should be regarded as an acceptable alternative.
© Pathak et al 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Urine testing for HPV.BMJ. 2014 Sep 16;349:g5542. doi: 10.1136/bmj.g5542. BMJ. 2014. PMID: 25230583 No abstract available.
-
Authors' reply to Vorsters and colleagues.BMJ. 2014 Oct 15;349:g6253. doi: 10.1136/bmj.g6253. BMJ. 2014. PMID: 25319367 No abstract available.
-
Urine testing for HPV: rationale for using first void.BMJ. 2014 Oct 15;349:g6252. doi: 10.1136/bmj.g6252. BMJ. 2014. PMID: 25319476 No abstract available.
References
-
- Einstein MH, Schiller JT, Viscidi RP, Strickler HD, Coursaget P, Tan T, et al. Clinician’s guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis 2009;9:347-56. - PubMed
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007;121:621-32. - PubMed
-
- Trent Cancer Registry. Profile of cervical cancer in england: incidence, mortality and survival. National Cancer Intelligence Network, 2012.
-
- National Cancer Intelligence Network. Cervical cancer incidence and screening coverage. 2014. www.ncin.org.uk/publications/data_briefings/cervical_incidence_and_scree....
-
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012;62:147-72. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical