Endogenous polyamine function--the RNA perspective
- PMID: 25232095
- PMCID: PMC4191411
- DOI: 10.1093/nar/gku837
Endogenous polyamine function--the RNA perspective
Abstract
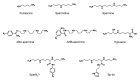
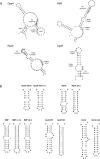
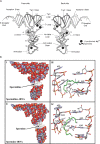
Recent progress with techniques for monitoring RNA structure in cells such as 'DMS-Seq' and 'Structure-Seq' suggests that a new era of RNA structure-function exploration is on the horizon. This will also include systematic investigation of the factors required for the structural integrity of RNA. In this context, much evidence accumulated over 50 years suggests that polyamines play important roles as modulators of RNA structure. Here, we summarize and discuss recent literature relating to the roles of these small endogenous molecules in RNA function. We have included studies directed at understanding the binding interactions of polyamines with polynucleotides, tRNA, rRNA, mRNA and ribozymes using chemical, biochemical and spectroscopic tools. In brief, polyamines bind RNA in a sequence-selective fashion and induce changes in RNA structure in context-dependent manners. In some cases the functional consequences of these interactions have been observed in cells. Most notably, polyamine-mediated effects on RNA are frequently distinct from those of divalent cations (i.e. Mg2+) confirming their roles as independent molecular entities which help drive RNA-mediated processes.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Tabor H., Tabor C.W. Spermidine, spermine, and related amines. Pharmacol. Rev. 1964;16:245–300. - PubMed
-
- Agostinelli E., Marques M.P., Calheiros R., Gil F.P., Tempera G., Viceconte N., Battaglia V., Grancara S., Toninello A. Polyamines: fundamental characters in chemistry and biology. Amino Acids. 2010;38:393–403. - PubMed
-
- Iitaka Y., Huse Y. The crystal structure of spermine phosphate hexahydrate. Acta Crystallogr. 1965;18:110–121. - PubMed
-
- Giglio E., Liquori A.M., Puliti R., Ripamonti A. The crystal structure of spermine tetrahydrochloride. Acta Crystallogr. 1966;20:652–659.
-
- Giglio E., Liquori A.M., Puliti R., Ripamonti A. The crystal structure of spermidine trihydrochloride. Acta Crystallogr. 1966;29:683–688.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

