Emergent exploration via novelty management
- PMID: 25232104
- PMCID: PMC6705324
- DOI: 10.1523/JNEUROSCI.1872-14.2014
Emergent exploration via novelty management
Abstract
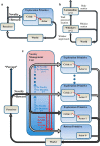
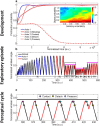
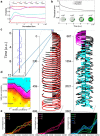
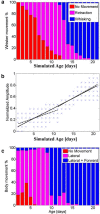
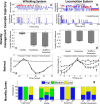
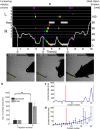
When encountering novel environments, animals perform complex yet structured exploratory behaviors. Despite their typical structuring, the principles underlying exploratory patterns are still not sufficiently understood. Here we analyzed exploratory behavioral data from two modalities: whisking and locomotion in rats and mice. We found that these rodents maximized novelty signal-to-noise ratio during each exploration episode, where novelty is defined as the accumulated information gain. We further found that these rodents maximized novelty during outbound exploration, used novelty-triggered withdrawal-like retreat behavior, and explored the environment in a novelty-descending sequence. We applied a hierarchical curiosity model, which incorporates these principles, to both modalities. We show that the model captures the major components of exploratory behavior in multiple timescales: single excursions, exploratory episodes, and developmental timeline. The model predicted that novelty is managed across exploratory modalities. Using a novel experimental setup in which mice encountered a novel object for the first time in their life, we tested and validated this prediction. Further predictions, related to the development of brain circuitry, are described. This study demonstrates that rodents select exploratory actions according to a novelty management framework and suggests a plausible mechanism by which mammalian exploration primitives can be learned during development and integrated in adult exploration of complex environments.
Keywords: active sensing; hierarchical model; intrinsic motivation; reinforcement learning; whisker system.
Copyright © 2014 the authors 0270-6474/14/3412646-16$15.00/0.
Figures
References
-
- Baldassarre G. What are intrinsic motivations? A biological perspective. Paper presented at IEEE International Conference on Development and Learning (ICDL); August; Frankfurt am Main, Germany. 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
