The visual input to the retina during natural head-free fixation
- PMID: 25232108
- PMCID: PMC4166157
- DOI: 10.1523/JNEUROSCI.0229-14.2014
The visual input to the retina during natural head-free fixation
Abstract
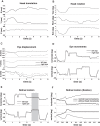
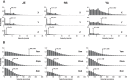
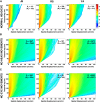
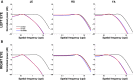
Head and eye movements incessantly modulate the luminance signals impinging onto the retina during natural intersaccadic fixation. Yet, little is known about how these fixational movements influence the statistics of retinal stimulation. Here, we provide the first detailed characterization of the visual input to the human retina during normal head-free fixation. We used high-resolution recordings of head and eye movements in a natural viewing task to examine how they jointly transform spatial information into temporal modulations. In agreement with previous studies, we report that both the head and the eyes move considerably during fixation. However, we show that fixational head and eye movements mostly compensate for each other, yielding a spatiotemporal redistribution of the input power to the retina similar to that previously observed under head immobilization. The resulting retinal image motion counterbalances the spectral distribution of natural scenes, giving temporal modulations that are equalized in power over a broad range of spatial frequencies. These findings support the proposal that "ocular drift," the smooth fixational motion of the eye, is under motor control, and indicate that the spatiotemporal reformatting caused by fixational behavior is an important computational element in the encoding of visual information.
Keywords: eye movements; head movements; microsaccade; ocular drift; retina; visual fixation.
Copyright © 2014 the authors 0270-6474/14/3412701-15$15.00/0.
Figures






References
-
- Atick JJ, Redlich AN. What does the retina know about natural scenes? Neural Comput. 1992;4:196–210. doi: 10.1162/neco.1992.4.2.196. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
