Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling
- PMID: 25232121
- PMCID: PMC4166165
- DOI: 10.1523/JNEUROSCI.1494-14.2014
Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling
Abstract
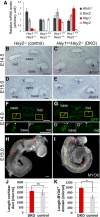
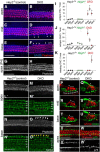
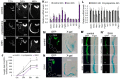
Mechano-sensory hair cells (HCs), housed in the inner ear cochlea, are critical for the perception of sound. In the mammalian cochlea, differentiation of HCs occurs in a striking basal-to-apical and medial-to-lateral gradient, which is thought to ensure correct patterning and proper function of the auditory sensory epithelium. Recent studies have revealed that Hedgehog signaling opposes HC differentiation and is critical for the establishment of the graded pattern of auditory HC differentiation. However, how Hedgehog signaling interferes with HC differentiation is unknown. Here, we provide evidence that in the murine cochlea, Hey1 and Hey2 control the spatiotemporal pattern of HC differentiation downstream of Hedgehog signaling. It has been recently shown that HEY1 and HEY2, two highly redundant HES-related transcriptional repressors, are highly expressed in supporting cell (SC) and HC progenitors (prosensory cells), but their prosensory function remained untested. Using a conditional double knock-out strategy, we demonstrate that prosensory cells form and proliferate properly in the absence of Hey1 and Hey2 but differentiate prematurely because of precocious upregulation of the pro-HC factor Atoh1. Moreover, we demonstrate that prosensory-specific expression of Hey1 and Hey2 and its subsequent graded downregulation is controlled by Hedgehog signaling in a largely FGFR-dependent manner. In summary, our study reveals a critical role for Hey1 and Hey2 in prosensory cell maintenance and identifies Hedgehog signaling as a novel upstream regulator of their prosensory function in the mammalian cochlea. The regulatory mechanism described here might be a broadly applied mechanism for controlling progenitor behavior in the central and peripheral nervous system.
Keywords: Atoh1; Hedgehog signaling; Hey1; Hey2; hair cells; inner ear cochlea.
Copyright © 2014 the authors 0270-6474/14/3412865-12$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
