Identification of a novel RNA virus lethal to tilapia
- PMID: 25232154
- PMCID: PMC4313277
- DOI: 10.1128/JCM.00827-14
Identification of a novel RNA virus lethal to tilapia
Abstract
Tilapines are important for the sustainability of ecological systems and serve as the second most important group of farmed fish worldwide. Significant mortality of wild and cultured tilapia has been observed recently in Israel. The etiological agent of this disease, a novel RNA virus, is described here, and procedures allowing its isolation and detection are revealed. The virus, denominated tilapia lake virus (TiLV), was propagated in primary tilapia brain cells or in an E-11 cell line, and it induced a cytopathic effect at 5 to 10 days postinfection. Electron microscopy revealed enveloped icosahedral particles of 55 to 75 nm. Low-passage TiLV, injected intraperitoneally in tilapia, induced a disease resembling the natural disease, which typically presents with lethargy, ocular alterations, and skin erosions, with >80% mortality. Histological changes included congestion of the internal organs (kidneys and brain) with foci of gliosis and perivascular cuffing of lymphocytes in the brain cortex; ocular inflammation included endophthalmitis and cataractous changes of the lens. The cohabitation of healthy and diseased fish demonstrated that the disease is contagious and that mortalities (80 to 100%) occur within a few days. Fish surviving the initial mortality were immune to further TiLV infections, suggesting the mounting of a protective immune response. Screening cDNA libraries identified a TiLV-specific sequence, allowing the design of a PCR-based diagnostic test. This test enables the specific identification of TiLV in tilapines and should help control the spread of this virus worldwide.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


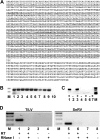
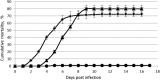
Similar articles
-
Establishment and characterization of a cell line from tilapia brain for detection of tilapia lake virus.J Fish Dis. 2018 Dec;41(12):1803-1809. doi: 10.1111/jfd.12889. Epub 2018 Oct 15. J Fish Dis. 2018. PMID: 30320411
-
Detection of Tilapia Lake Virus in Clinical Samples by Culturing and Nested Reverse Transcription-PCR.J Clin Microbiol. 2017 Mar;55(3):759-767. doi: 10.1128/JCM.01808-16. Epub 2016 Dec 14. J Clin Microbiol. 2017. PMID: 27974544 Free PMC article.
-
Combining Phage Display Technology with In Silico-Designed Epitope Vaccine to Elicit Robust Antibody Responses against Emerging Pathogen Tilapia Lake Virus.J Virol. 2023 Apr 27;97(4):e0005023. doi: 10.1128/jvi.00050-23. Epub 2023 Mar 28. J Virol. 2023. PMID: 36975794 Free PMC article.
-
Tilapia lake virus: The story so far.J Fish Dis. 2020 Oct;43(10):1115-1132. doi: 10.1111/jfd.13237. Epub 2020 Aug 23. J Fish Dis. 2020. PMID: 32829488 Review.
-
Susceptibilities of ten fish cell lines to infection with Tilapia lake virus.Microb Pathog. 2022 May;166:105510. doi: 10.1016/j.micpath.2022.105510. Epub 2022 Apr 11. Microb Pathog. 2022. PMID: 35421555 Review.
Cited by
-
ICTV Virus Taxonomy Profile: Amnoonviridae 2023.J Gen Virol. 2023 Oct;104(10):001903. doi: 10.1099/jgv.0.001903. J Gen Virol. 2023. PMID: 37873742 Free PMC article.
-
Knowns and unknowns of TiLV-associated neuronal disease.Virulence. 2024 Dec;15(1):2329568. doi: 10.1080/21505594.2024.2329568. Epub 2024 Mar 31. Virulence. 2024. PMID: 38555518 Free PMC article. Review.
-
Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer.Vaccines (Basel). 2022 Jan 21;10(2):167. doi: 10.3390/vaccines10020167. Vaccines (Basel). 2022. PMID: 35214626 Free PMC article.
-
The snakehead retrovirus promoter functions independently of the 3'ORF protein and its products are maternally inherited in transgenic zebrafish.PLoS Pathog. 2025 Jun 12;21(6):e1013243. doi: 10.1371/journal.ppat.1013243. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40504871 Free PMC article.
-
Concentration and quantification of Tilapia tilapinevirus from water using a simple iron flocculation coupled with probe-based RT-qPCR.PeerJ. 2022 Apr 18;10:e13157. doi: 10.7717/peerj.13157. eCollection 2022. PeerJ. 2022. PMID: 35462762 Free PMC article.
References
-
- Food and Agriculture Organization of the United Nations (FAO). 2010. Cultured aquatic species information programme, Oreochromis niloticus (Linnaeus, 1758). Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/fishery/culturedspecies/Oreochromis_niloticus/en.
-
- Food and Agriculture Organization of the United Nations (FAO). 2010. Fisheries and Aquaculture Department. Species fact sheets: Oreochromis niloticus (Linnaeus, 1758). Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/fishery/species/3217/en.
-
- Food and Agriculture Organization of the United Nations (FAO). 2004. The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/sof/sofia/index_en.htm.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

