Otilonium bromide in irritable bowel syndrome: a dose-ranging randomized double-blind placebo-controlled trial
- PMID: 25232263
- PMCID: PMC4161814
- DOI: 10.3748/wjg.v20.i34.12283
Otilonium bromide in irritable bowel syndrome: a dose-ranging randomized double-blind placebo-controlled trial
Abstract
Aim: To examine the efficacy and safety of otilonium bromide (OB) in treatment-sensitive functional irritable bowel syndrome (IBS) clinical parameters.
Methods: Ninety-three patients (44.8 ± 12.6 years, 69% female) with IBS symptoms complying with Rome II criteria participated in this double-blind, placebo-controlled, randomised, dose-ranging phase I/II study. Patients were administered OB 20 mg (n = 24), 40 mg (n = 23) and 80 mg (n = 23) tid or placebo (n = 23) in 4 parallel groups for 4 wk. Primary efficacy variables included abdominal discomfort, intestinal habits, number of daily evacuations and stool consistency. Secondary efficacy measures included return to regular intestinal habits and global discomfort. Safety was also assessed.
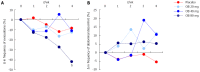
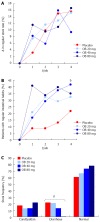
Results: Baseline clinical characteristics were similar among the 4 groups. Although individual parameters such as intensity and frequency of abdominal discomfort, bloating or pain were reduced by OB over the 4 wk, no significant differences were observed between groups. Similarly, no difference was observed between OB treatment or placebo for mucus in stool and incomplete or difficulty of evacuation. However, evacuation frequency was significantly reduced after 4 wk by 80 mg OB compared to placebo (-8.36% for placebo vs -41.9% for 80 mg OB, P < 0.01). While 21.7% of patients in the placebo group experienced regular intestinal habits after 4 wk, this improvement was greater for patients treated with 40 mg OB (P < 0.01 vs placebo). Furthermore, a dose-dependent reduction in frequency of diarrhoea (χ(2)-test for trend = 11.5, P < 0.001) and an increase in normal stool frequency was observed. Combining individual variables into a global discomfort index revealed significant improvement among increasing OB doses, favouring 40 mg (P = 0.013) and 80 mg OB (P = 0.001) over placebo. No difference was observed between frequency of adverse events for placebo vs OB.
Conclusion: This dose-ranging study demonstrates that OB at 40 and 80 mg can improve individual and global clinical symptoms of IBS compared to placebo over a 4-wk period.
Keywords: Acute treatment; Irritable bowel syndrome; Otilonium bromide; Spasmolytic.
Figures


Similar articles
-
Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome.Aliment Pharmacol Ther. 2011 Aug;34(4):432-42. doi: 10.1111/j.1365-2036.2011.04730.x. Epub 2011 Jun 16. Aliment Pharmacol Ther. 2011. PMID: 21679214 Clinical Trial.
-
Efficacy and mode of action of mesalazine in the treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D): study protocol for a randomised controlled trial.Trials. 2013 Jan 9;14:10. doi: 10.1186/1745-6215-14-10. Trials. 2013. PMID: 23302220 Free PMC article. Clinical Trial.
-
A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS.Am J Gastroenterol. 2005 Jan;100(1):115-23. doi: 10.1111/j.1572-0241.2005.40365.x. Am J Gastroenterol. 2005. PMID: 15654790 Clinical Trial.
-
Irritable bowel syndrome: focus on otilonium bromide.Expert Rev Gastroenterol Hepatol. 2014 Feb;8(2):131-7. doi: 10.1586/17474124.2014.869477. Expert Rev Gastroenterol Hepatol. 2014. PMID: 24417261 Review.
-
Role of antispasmodics in the treatment of irritable bowel syndrome.World J Gastroenterol. 2014 May 28;20(20):6031-43. doi: 10.3748/wjg.v20.i20.6031. World J Gastroenterol. 2014. PMID: 24876726 Free PMC article. Review.
Cited by
-
Antispasmodics for Chronic Abdominal Pain: Analysis of North American Treatment Options.Am J Gastroenterol. 2021 Aug 1;116(8):1587-1600. doi: 10.14309/ajg.0000000000001266. Am J Gastroenterol. 2021. PMID: 33993133 Free PMC article. Review.
-
Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis.Therap Adv Gastroenterol. 2017 Mar;10(3):311-322. doi: 10.1177/1756283X16681708. Epub 2017 Jan 16. Therap Adv Gastroenterol. 2017. PMID: 28246548 Free PMC article.
-
Effect of herbal extract granules combined with otilonium bromide on irritable bowel syndrome with diarrhoea: a study protocol for a randomised controlled trial.BMJ Open. 2017 Dec 1;7(11):e018362. doi: 10.1136/bmjopen-2017-018362. BMJ Open. 2017. PMID: 29196484 Free PMC article. Clinical Trial.
-
Effect of Samryungbaekchul-san Combined with Otilonium Bromide on Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Randomized Controlled Trial.J Clin Med. 2019 Sep 27;8(10):1558. doi: 10.3390/jcm8101558. J Clin Med. 2019. PMID: 31569833 Free PMC article.
-
Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment.World J Gastroenterol. 2023 Jul 14;29(26):4120-4135. doi: 10.3748/wjg.v29.i26.4120. World J Gastroenterol. 2023. PMID: 37475846 Free PMC article. Review.
References
-
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. - PubMed
-
- Mearin F, Badía X, Balboa A, Baró E, Caldwell E, Cucala M, Díaz-Rubio M, Fueyo A, Ponce J, Roset M, et al. Irritable bowel syndrome prevalence varies enormously depending on the employed diagnostic criteria: comparison of Rome II versus previous criteria in a general population. Scand J Gastroenterol. 2001;36:1155–1161. - PubMed
-
- Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. - PubMed
-
- Sperber AD, Shvartzman P, Friger M, Fich A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the ‘true’ prevalence of irritable bowel syndrome? Eur J Gastroenterol Hepatol. 2007;19:441–447. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

