Efficacy of immunosuppression monotherapy after liver transplantation: a meta-analysis
- PMID: 25232269
- PMCID: PMC4161820
- DOI: 10.3748/wjg.v20.i34.12330
Efficacy of immunosuppression monotherapy after liver transplantation: a meta-analysis
Abstract
Aim: To assess the advantages and disadvantages of immunosuppression monotherapy after transplantation and the impact of monotherapy on hepatitis C virus (HCV) recurrence.
Methods: Articles from Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded, including non-English literature identified in these databases, were searched up to January 2013. We included randomized clinical trials comparing various immunosuppression monotherapy and prednisone-based immunosuppression combinations for liver transplantation. The modified Jadad scale score or the Oxford quality scoring system was used. Meta-analyses were performed with weighted random-effects models.
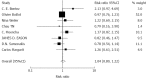
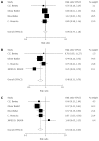
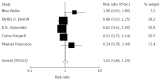
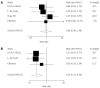
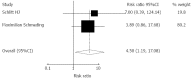
Results: A total of 14 randomized articles including 1814 patients were identified. Eight trials including 1214 patients compared tacrolimus monotherapy (n = 610) vs tacrolimus plus steroids or triple therapy regarding acute rejection and adverse events (n = 604). Five trials, including 285 patients, compared tacrolimus monotherapy (n = 143) vs tacrolimus plus steroids or triple therapy regarding hepatitis C recurrence (n = 142). Four trials including 273 patients compared cyclosporine monotherapy (n = 148) vs cyclosporine and steroids regarding acute rejection and adverse events (n = 125). Two trials including 170 patients compared mycophenolate mofetil monotherapy (n = 86) vs combinations regarding acute rejection (n = 84). There were no significant differences in the acute rejection rates between tacrolimus monotherapy (RR = 1.04, P = 0.620), and cyclosporine monotherapy (RR = 0.89, P = 0.770). Mycophenolate mofetil monotherapy had a significant increase in the acute rejection rate (RR = 4.50, P = 0.027). Tacrolimus monotherapy had no significant effects on the recurrence of hepatitis C (RR = 1.03, P = 0.752). More cytomegalovirus infection (RR = 0.48, P = 0.000) and drug-related diabetes mellitus (RR = 0.54, P = 0.000) were observed in the immunosuppression combination therapy groups.
Conclusion: Tacrolimus and cyclosporine monotherapy may be as effective as immunosuppression combination therapy. Mycophenolate mofetil monotherapy was not considerable. Tacrolimus monotherapy does not increase recurrence of HCV.
Keywords: Cytomegalovirus; Diabetes; Immunosuppression monotherapy; Liver transplantation; Meta-analysis.
Figures

Comment in
-
Duplicate publication bias weakens the validity of meta-analysis of immunosuppression after transplantation.World J Gastroenterol. 2017 Oct 21;23(39):7198-7200. doi: 10.3748/wjg.v23.i39.7198. World J Gastroenterol. 2017. PMID: 29093629 Free PMC article.
References
-
- Bonaccorsi-Riani E, Sempoux C, Piette N, Julliard O, Kabamba B, Ciccarelli O, Roggen F, De Reyck C, Hassoun Z, Lerut J. Impact of steroid-avoidance immunosuppression on long-term outcome after liver transplantation for HCV cirrhosis: the need for well documented long-term follow-up. Acta Gastroenterol Belg. 2012;75:411–418. - PubMed
-
- Stegall MD, Everson G, Schroter G, Bilir B, Karrer F, Kam I. Metabolic complications after liver transplantation. Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation. 1995;60:1057–1060. - PubMed
-
- Jindal RM, Sidner RA, Hughes D, Pescovitz MD, Leapman SB, Milgrom ML, Lumeng L, Filo RS. Metabolic problems in recipients of liver transplants. Clin Transplant. 1996;10:213–217. - PubMed
-
- Marubashi S, Umeshita K, Asahara T, Fujiwara K, Haga H, Hashimoto T, Hatakeyama K, Ichida T, Kanematsu T, Kitajima M, et al. Steroid-free living donor liver transplantation for HCV--a multicenter prospective cohort study in Japan. Clin Transplant. 2012;26:857–867. - PubMed
-
- Goddard S, Adams DH. Methylprednisolone therapy for acute rejection: too much of a good thing? Liver Transpl. 2002;8:535–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

